Prepare potassium cyanide. Potassium cyanide - poisoning with a dangerous substance
Potassium cyanide is the most infamous poison. He gained his fame thanks to the authors of detective novels, who often "used" this poisonous substance in their works. However, in nature there are poisons that act much faster and more efficiently than potassium cyanide. Obviously, the popularity of this substance is also due to the availability of acquisition on turn of XIX-XX centuries, when it could be easily purchased at any pharmacy. But what are cyanides today? What types of toxic substances from this family exist? Where are they used and is it possible to get poisoned with this poison today? It is these questions that will be discussed in this article.
What it is
Potassium cyanide is a chemical compound derived from hydrocyanic acid. The cyanide formula is KCN. This substance was first obtained by the Swedish chemist Carl Wilhelm Scheele in 1782, and in the middle of the 19th century, the German chemist Robert Wilhelm Bunsen developed a method for the industrial synthesis of poison. It was assumed that this substance would not be used for the purpose of killing their own kind, but for the control of agricultural pests and in the leather industry. Derivatives of hydrocyanic acid were often used as a coloring pigment in paints.

Nevertheless, at the beginning of the 20th century, the French military first used cyanides as chemical weapon. Despite the fact that the gas attack in the battles on the banks of the Seine did not bring the expected result, some German scientists considered the "prospects" of using cyanide in military operations. During the Second World War, the Nazis were already widely using more advanced modifications of poisonous substances created on the basis of cyanides in concentration camps and on some sectors of the front.
Types of cyanides
What is potassium cyanide and what effect it has on the human body, most people probably know. However, few people know that poisonous family may contain both organic and inorganic cyanides.
The first group is mainly used in pharmacology and agriculture(in the fight against harmful insects). The second group found wide application in the chemical industry and photo printing, leather and textile production, as well as in mining and electroplating.

What does it look like
People who know what cyanide is describe it as a translucent powder with a crystalline structure. This substance is completely soluble in water. However, due to the fact that stronger acids can easily displace hydrocyanic acid from the compound, this toxic substance is considered an extremely unstable compound. As a result of the ongoing reactions, the elements of the CN cyano group volatilize, so the original compound loses its toxic properties. Humid air can have a negative effect on the toxic effect.
Smell
Potassium cyanide is believed to have specific smell rancid almonds, however, not all people are able to catch it. This is due to the individual characteristics of the olfactory apparatus of each person.
Where is cyanide found?
What is cyanide in nature and where can it be found? AT pure form potassium cyanide does not exist in nature, however, poisonous compounds of cyano groups - amygdalins, can be found in apricot, cherry, peach and plum pits. They can be found in almonds. Elderberry leaves and shoots also contain amygdalin.
The danger to the human body when using these products is hydrocyanic acid, which is formed during the breakdown of amygdalin. Death can occur after consuming just one gram of the substance, which corresponds to about 100 grams of apricot kernels.
In everyday life, cyanide can be found in reagents used in darkrooms, as well as in preparations for cleaning jewelry. Some of this substance is used in insect traps. Cyanides are added to artistic paints with azure hues. Due to the interaction with iron, which is also part of gouache and watercolors, they give a deep blue color.

Risk of poisoning
Hydrocyanic acid salts and cyanides are very toxic substances that can cause severe forms of poisoning. The greatest likelihood of getting poisoned from the action of cyanide is in people working in mining and ore-dressing mines and in electroplating shops. Here, potassium or sodium cyanides are used in technological processes when metals are catalyzed.

People who are in the zone of toxic emissions from these enterprises also have the risk of getting poisoned by such toxic substances. Thus, on the territory of Romania and Hungary in the early 2000s, as a result of accidental discharges from mining and processing enterprises into the Danube River, residents of the floodplain neighborhood suffered.
At risk of getting toxic cyanide poisoning are employees of special laboratories in which these substances are used as reagents.
Human impact
Under the influence of poison, a cellular enzyme is blocked - cytochrome oxidase, which is responsible for the absorption of oxygen in the cell. As a result, the cells are filled with oxygen, but they cannot absorb it. This leads to the fact that in the body there is a violation of vital metabolic processes. The effect of such exposure is tantamount to suffocation.
Cyanides are poisonous when ingested with food or water, poisoning can be obtained as a result of inhalation of the vapors of the solution. Cyanides can penetrate through damaged skin.
Even in small quantities, they are extremely dangerous for the health of living organisms. Due to the high toxicity, the use of these drugs is controlled with particular rigor.

Symptoms of poisoning
A mild form of cyanide poisoning is accompanied by a sore throat, dizziness, drooling, vomiting, and a panic attack. In more severe forms, bitterness in the mouth increases, heart pains appear, the person loses consciousness, convulsions and paralysis of the respiratory tract begin. Severe poisoning is usually accompanied by uncontrolled urinary incontinence and bowel movements, excessive redness of the skin and mucous membranes. After these manifestations comes death.
First aid
To provide adequate assistance, it is first necessary to establish how the poison could enter the body of the victim. If the poisoning occurred through the skin, then it is necessary to change clothes, on which, most likely, particles of the poisonous substance remained. The victim himself must be wiped with soapy water.
If the poison has entered the body along with food, then first of all it is necessary to induce vomiting and rinse the stomach. To do this, you need to drink a large amount of water with the addition of potassium permanganate (potassium permanganate) or baking soda. After washing the stomach, the victim is given any sweet drink. To alleviate the symptoms of poisoning, the victim must be removed to fresh air.
If the victim is unconscious, it is necessary to monitor his heartbeat and breathing. In the absence of breathing, artificial respiration should be performed. However, a person conducting such events should exclude possible poisoning by poison vapors and seek medical help.
In any case, you need to call an ambulance. Only medical worker, who has special education and experience, can take adequate treatment measures. Arriving doctors must be informed that the cause of poisoning is hydrocyanic acid. In this case, the doctor will intravenously inject an antidote - sodium thiosulfate. The antidote helps to reduce the harmful effects of the poison. If necessary, the doctor will take resuscitation measures and hospitalize the victim for further treatment.
Antidotes
The lethal dose for humans is 17 mg per kilogram. total weight body. Death occurs just a few minutes after a sufficient amount of poison enters the body. However, this number is considered conditional. The degree of poisoning depends on the method of entry, physical features person and food. With regular ingestion of small doses of cyanide poison, poisoning occurs gradually, over a long time.

It has been proven that when cyanide enters the body, ordinary glucose is a kind of antidote to the toxic property of the substance. Sugar contributes to the instantaneous oxidation of hydrocyanic acid compounds and potassium salts. Therefore, people who come into contact with toxic compounds usually carry a few pieces of sugar with them. At the first symptoms of poisoning, they eat it to neutralize the action of poisonous compounds.

Potassium cyanide is a poison that replaced arsenic and has been used more than once in the murders of political figures. After the discovery of the features of white crystals, potassium cyanide was banned from free sale. According to toxicologists, the inorganic substance ranks fifth in the ranking of fast-acting poisons. When working with this chemical component, it is not enough to observe safety measures - you need to know the mechanism of action of the poison and be able to help the victim in time.
What is potassium cyanide?
Potassium cyanide - a derivative of hydrocyanic acid, is indicated by the chemical formula KCN. In a solid state of aggregation, it looks like a crystalline powder without color. This is an unstable compound, since hydrocyanic acid is a weak complex of ionic elements. The cyano group is displaced by any salts of stronger acids, which volatilizes in the form of vapors. The gaseous state becomes poisonous while the remainder is rendered harmless. Bonds are easily broken with concentrated glucose solution. heat treatment and in conditions of high humidity.
Types and characteristics

The toxic substance is found in peaches and 250 plum varieties. When eating fruits, poisoning does not occur, since the poison is contained in the bones. As a result of metabolism, amygdalin from the group of natural glycosides is cleaved under the action of of hydrochloric acid in the stomach, forming a toxin. The rest of the substance decomposes into glucose, benzaldehyde and hydrocyanic acid. Sugar instantly neutralizes the amount of cyanide formed, as a result of which nothing threatens human health.
Characteristics:
- By appearance resembles crystals of refined sugar.
- Cyanide is freely soluble in water, without affecting the color and density of the liquid.
- In the presence of poisonous vapors or crystals, a person feels a slight smell of almonds.
Olfactory receptors 50% of people recognize aroma. The peculiarity depends on the individual characteristics and the genetic factor. Due to the risk of poisoning, strong inhalation of air with toxic fumes is not recommended.
Where are cyanides found?
In nature, potassium cyanide crystals cannot be found. Hazardous substance is produced by cells poisonous plants. Present in small quantities in the bones:
- apricots;
- plums;
- peaches
- cherries.
Cyanide is used in the mining industry, jewelry, and in the manufacture of paints. Chemical poisoning threatens employees industrial enterprises, laboratory assistants and chemists. In the domestic sphere, the poisonous compound is found in photoreactives and insect pest control products.
Human exposure and risk of poisoning
There is a hypothesis that when crystals enter the stomach, death occurs instantly. The theory is confirmed only in 50% of clinical experiments on animals.
Potassium cyanide is dangerous to the human body, but the likelihood of instant death when taken orally is minimal. The principle of action of a chemical is difficult to understand and is divided in the scientific field into 4 stages of poisoning:

Studies have shown that death does not occur instantly. Due to the lack of oxygen, a person is able to lose consciousness, which others perceive as a fatal outcome. Within a minute, due to the cessation of the diaphragm, breathing is not felt, the heart refuses to generate nerve impulses. The pulse is thready. 5 minutes after breathing and heartbeat stops, the body dies completely.
A toxic compound is able to penetrate the body not only when administered orally, but also by inhaling the gaseous state, when the poison enters the body through the skin by diffusion, or when it enters the bloodstream through wounds.
Symptoms
In 85% of cases, poisoning takes a chronic or acute form. In the latter case, signs of poisoning appear 2–3 minutes after the use of potassium cyanide in food or when inhaled in the form of a vapor or powder.
The rapid action is due to the penetration of a chemical compound into the blood vessels through the mucous membranes of the oral cavity, esophagus, through the walls of the stomach during the digestive act.
In the 4 phases of poisoning, different symptoms are observed:
| Stage name | Signs of poisoning |
| Prodromal (beginning of symptoms of poisoning) |
|
| Active process of oxygen starvation |
|
| Cell necrosis |
|
| Fatal outcome | After cellular respiration stops, death occurs within 5–20 minutes, depending on the dose taken. |
A person begins to feel symptoms after 40 minutes if the dosage is small. The concentration in the blood does not reach a lethal level, and the liver cells cope with the neutralization of the poison.
Chronic cyanide poisoning is milder. Intoxication lasts for several days: toxic substances accumulate, gradually weakening the body. Every day the risk of death increases. Symptoms begin to appear slowly.

Potassium cyanide circulates in the blood for up to 4 hours. If a fatal outcome does not occur during this period, the body begins to excrete the poison, and the person survives. Poisoning leaves its mark: there is a violation of the activity of the brain due to the death of neurons as a result of oxygen starvation. Lost connections cannot be restored.
First aid and treatment
At the first symptoms of intoxication, it is necessary to call an ambulance team, and then provide prompt first aid:
- Provide access to fresh air. If a person is poisoned by vapors - free from squeezing clothing.
- If a poisonous compound enters through the mouth, it is necessary to rinse the stomach large quantity water, soda, a weak solution of potassium permanganate.
- If there is no consciousness, the pulse is not felt, and breathing has stopped, resuscitation measures are taken. Two breaths mouth to mouth artificial ventilation lungs alternate with 30 quick presses in the region of the heart.
- The toxin is able to penetrate the skin if it soaks into clothing. Toxic tissue must be removed to prevent further intoxication.
In a medical institution, specialists determine the degree of poisoning and administer an antidote to neutralize potassium cyanide. Analysis and therapy with medicines, including an antidote, are prescribed. In a difficult situation, the patient is hospitalized and the cyanide is removed gradually in the conditions of inpatient treatment.
Medicinal assistance is provided with the help of nitrogen-containing drugs and substances that release sulfur radicals from methemoglobin formers. Groups of medicines converge in the mechanism of action - they contribute to the separation of oxygen molecules from hemoglobin, restoring the respiratory process in cells. In practice, use:

- vapors of amyl nitrite;
- intravenous solution of sodium nitrite;
- methylene blue solution.
An unexpected discovery in early XXI in. - antidote against potassium cyanide (glucose). Sugar was the reason for the failure of a number of attempts on Rasputin and the elephant Yambo, as the killers put poison in confectionery. If cyanide has already entered the body, eating glucose is useless. The monosaccharide is able to weaken the action of the toxin only when direct contact as a result of a fusion reaction. Sulfur has a similar property, the molecules of which neutralize the poison in the stomach.
An increase in the concentration of glucose in the blood plasma after a meal helps to neutralize the toxin in the vessels.
With signs of chronic intoxication, it is necessary to stop contact with a toxic substance and undergo a medical examination.
Prevention
Acute poisoning with one of the deadly poisons disrupts the activity of the brain and can lead to death. 85% of cases of potassium cyanide poisoning occur in laboratory workers and the mining industry. Employees of relevant professions must comply with safety precautions:
- In case of leakage of vapors and violation of the tightness of the equipment, it is required to leave the room immediately.
- It is necessary to work only in special protective suits.
- Before interacting with the toxin, you need to wear glasses, because the poisonous compound can settle on contact lenses.
- It is required to store an antidote in the first-aid kit of the working room.
- It is necessary to be able to provide first aid and know resuscitation actions.
With a slow effect of the poison on the body, chronic diseases become aggravated, the work of organs and systems is disrupted. It is not recommended to work with cyanide or try to obtain the substance at home. It is not known what dosage of poison a person can receive by inhalation or direct contact. There is a high chance of death, so extreme caution must be exercised.
From the gold mining enterprise into the Sekisovka River in the East Kazakhstan region, the maximum permissible concentration (MPC) of cyanide was exceeded by more than 500 times, the press service of the Ministry of Emergency Situations of Kazakhstan reported on Wednesday, November 2.
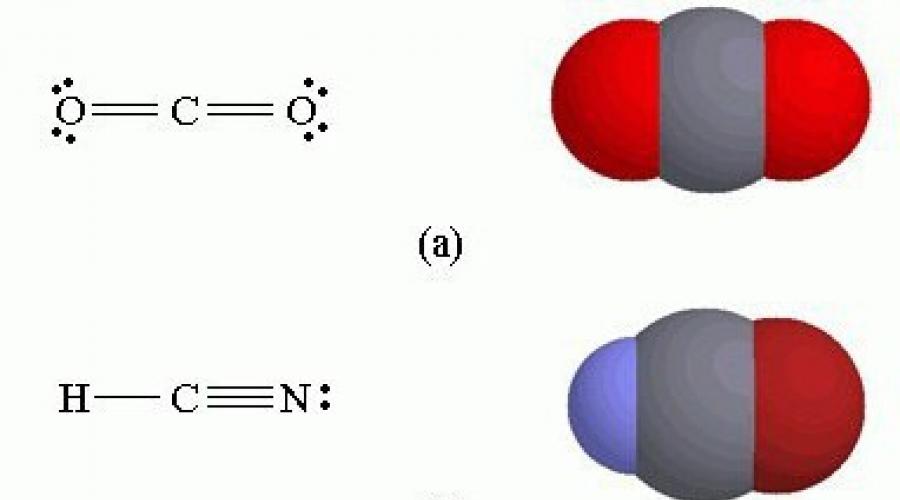
Cyanides include a large group of chemical compounds derived from hydrocyanic (cyanic) acid. All of them contain a cyano group - CN. There are inorganic cyanides (hydrocyanic acid, sodium and potassium cyanides, cyanide, cyanogen chloride, cyanogen bromide, calcium cyanide) and organic cyanides (cyanoformic and cyanoacetic acid esters, nitriles, thiocyanates, glycoside-amygdalin, etc.).
Inorganic cyanides are widely used in the chemical, leather, textile, photography, agriculture, gold mining and electroforming industries.
Organic cyanides are used for pest control in agriculture, organic synthesis, pharmaceutical industry, etc.
Hydrocyanic acid and its salts, cyanides, are among the most toxic substances and cause severe poisoning.
Hydrocyanic acid (HCN) is a light, volatile liquid with a characteristic bitter almond odor. It is a very strong poison: in the amount of 0.05 grams, it already causes fatal poisoning in humans.
Sodium and potassium cyanides are colorless crystals; they easily decompose in air in the presence of moisture with the release of hydrocyanic acid. Cyanogen chloride is a colorless liquid with a pungent odor. Bromine cyanide - colorless crystals with a pungent odor. Calcium cyanamide - pure white, technical - grayish-black fine powder. Cyanplava is a mixture of cyanides and chlorides of calcium and sodium, a dark gray powder (grains or crystals) with a slight smell of bitter almonds.
Cyanides enter the body through the digestive organs, respiratory organs, and rarely through the skin. The poisonous effect of cyanides is based on the fact that they bind to tissue enzymes responsible for cellular respiration, inhibiting their activity, and cause oxygen starvation of tissues.
Cyanide anions form complexes with ions ferrous iron, which leads to blockade of oxygen transfer to tissues and causes tissue hypoxia (oxygen starvation). As a result, the functions of the brain and respiratory center are disrupted.
When inhaled vapors of hydrocyanic acid, death occurs within one minute. Oral ingestion of sodium or potassium cyanide can also cause death in humans within minutes.
The action of potassium and sodium cyanides on the skin can cause the formation of cracks, the development of eczema.
The clinical picture of acute cyanide poisoning depends on the dose of the poison or the concentration of hydrocyanic acid vapor.
Symptoms of hydrocyanic acid poisoning
With mild poisoning: the smell of bitter almonds from the mouth, sore throat, dizziness, salivation, vomiting, fear, shock.
In severe poisoning: loss of consciousness, convulsions, hyperemia (overflow of blood vessels of the circulatory system) of the skin, paralysis of the respiratory center.
First aid
If you suspect hydrocyanic acid poisoning, first of all, the victim must be vomited, then taken to fresh air, given activated charcoal to drink and call an ambulance. When calling an ambulance, it is imperative to report that hydrocyanic acid poisoning has occurred.
The doctor must intravenously inject an antidote (antidote) of hydrocyanic acid - sodium thiosulfate, which weakens the effect of the poison. In case of violation of vital functions, the doctor takes resuscitation measures. After providing first aid, he hospitalizes the patient for further treatment.
The material was prepared on the basis of information from open sources
DAMAGES BY POISONING SUBSTANCES OF GENERAL TOXIC ACTION: PRUSIAN ACID AND POTASSIUM CYANIDE
Hydrocyanic acid and potassium cyanide are toxic substances of general toxic action, as well as sodium, cyanogen chloride, cyanogen bromide, carbon monoxide.
For the first time, hydrocyanic acid was synthesized by the Swedish scientist Karl Scheele in 1782. History knows cases of the use of cyanides for mass destruction of people. During the First World War (1916 on the Somme River), the French army used hydrocyanic acid as a poisonous substance, in the Nazi extermination camps the Nazis (1943-1945) used poisonous gases, cyclones (cyanoformic acid esters), American troops in South Vietnam (1963) used toxic organic cyanides (CS-type gases) against civilians. It is also known that in the United States the death penalty by poisoning convicts with hydrocyanic acid vapors in a special chamber.
Due to their high chemical activity and the ability to interact with numerous compounds of various classes, cyanides are widely used in many industries, agriculture, and scientific research, and this creates many opportunities for intoxication.
Thus, hydrocyanic acid and a large number of its derivatives are used in the extraction noble metals from ores, in galvanoplastic gilding and silvering, in the production of aromatic substances, chemical fibers, plastics, rubber, organic glass, plant growth stimulants, herbicides. Cyanides are also used as insecticides, fertilizers and defoliants. Hydrocyanic acid is released in a gaseous state at many production processes. There may also be cyanide poisoning due to the consumption of large amounts of seeds of almonds, peaches, apricots, cherries, plums and other plants of the Rosaceae family or tinctures from their fruits. It turned out that they all contain amygdalin glycoside, which decomposes in the body under the influence of the emulsin enzyme to form hydrocyanic acid, benzaldehyde and 2 glucose molecules. The largest number amygdalin is found in bitter almonds (up to 3%) and apricot seeds (up to 2%).
Physico-chemical properties and toxicity of hydrocyanic acid
Hydrocyanic acid - HCN - is a colorless, easily boiling (at 26 ° C) liquid with the smell of bitter almonds, with a specific gravity of 0.7, freezes at - 13.4 C. Cyanide poisoning develops when inhaled vapors of a toxic substance, when absorbed through the skin and through the mouth. In wartime, the most probable is the inhalation route of their entry into the body. According to WHO, Lt50 of hydrocyanic acid is 2 g/min/m3. When poisoning through the mouth, lethal doses for humans are: HCN - 1 mg / kg, KCN - 2.5 mg / kg; NaSN - 1.8 mg/kg.
Mechanism of toxic action
The mechanism of action of hydrocyanic acid has been studied in some detail. It is a substance that causes oxygen starvation of the tissue type. At the same time, a high oxygen content is observed both in arterial and venous blood and thus a decrease in the arterio-venous difference, a sharp decrease in oxygen consumption by tissues with a decrease in the formation of carbon dioxide in them.
It has been established that cyanides interfere with redox processes in tissues, disrupting oxygen activation by cytochrome oxidase. (The lecturer can dwell in more detail on modern concepts of cellular respiration).
Hydrocyanic acid and its salts, dissolved in the blood, reach the tissues, where they interact with the ferric form of cytochrome oxidase iron. Having combined with cyanide, cytochrome oxidase loses the ability to transfer electrons to molecular oxygen. Due to the failure of the final link of oxidation, the entire respiratory chain is blocked and tissue hypoxia develops. Oxygen is delivered to the tissues in sufficient quantities with arterial blood, but is not absorbed by them and passes unchanged into the venous bed. At the same time, the processes of formation of macroergs necessary for the normal activity of various organs and systems are disrupted. Glycolysis is activated, that is, the exchange from aerobic to anaerobic is rebuilt. The activity of other enzymes - catalase, peroxidase, lactate dehydrogenase - is also suppressed.
The effect of cyanides on various organs and systems
Action on the nervous system. As a result of tissue hypoxia, developing under the influence of hydrocyanic acid, the functions of the central nervous system. Cyanides in toxic doses cause at the beginning the excitation of the central nervous system, and then its depression. In particular, at the beginning of intoxication, excitation of the respiratory and vasomotor centers is observed. This is manifested by a rise in blood pressure and the development of severe shortness of breath. An extreme form of excitation of the central nervous system are clonic-tonic convulsions. Pronounced excitation of the nervous system is replaced by paralysis (respiratory and vasomotor centers).
Action on the respiratory system. In the picture of acute poisoning, a pronounced increase in the frequency and depth of breathing is observed. Developing shortness of breath, apparently, should be considered as a compensatory reaction of the body to hypoxia. The stimulating effect of cyanides on respiration is due to the excitation of the chemoreceptors of the carotid sinus and the direct action of the poison on the cells of the respiratory center. The initial excitation of respiration, as intoxication develops, is replaced by its oppression up to a complete stop. The causes of these disorders are tissue hypoxia and depletion of energy resources in the cells of the carotid sinus and in the centers of the medulla oblongata.
Action on the cardiovascular system. In the initial period of intoxication, a slowing of the heart rate is observed. An increase in blood pressure and an increase in cardiac output occur due to the excitation of chemoreceptors of the carotid sinus and cells of the vasomotor center by cyanides, on the one hand, the release of catecholamines from the adrenal glands and, as a result, vasospasm, on the other. As poisoning progresses blood pressure falls, the pulse quickens, acute cardiovascular failure develops and cardiac arrest occurs.
Changes in the blood system. The content of erythrocytes in the blood increases, which is explained by the reflex contraction of the spleen in response to developing hypoxia. The color of venous blood becomes bright scarlet due to the excess oxygen content not absorbed by the tissues. The arterio-venous difference in oxygen sharply decreases. When tissue respiration is suppressed, both the gas and biochemical composition of the blood changes. The content of CO2 in the blood decreases due to less formation and increased release during hyperventilation. This leads at the beginning of the development of intoxication to gas alkalosis, which changes to metabolic acidosis, which is a consequence of the activation of glycolysis processes. Unoxidized metabolic products accumulate in the blood. The content of lactic acid increases, the content of acetone bodies increases, hyperglycemia is noted. The development of hypothermia is explained by the violation of redox processes in tissues. Thus, hydrocyanic acid and its salts cause the phenomena of tissue hypoxia and associated respiratory, circulatory, metabolic, and central nervous system disorders, the severity of which depends on the severity of intoxication.
CLINICAL PICTURE OF CYANIDE POISONING
Cyanide poisoning is characterized by the early onset of signs of intoxication, the rapid development of oxygen starvation, the predominant lesion of the central nervous system and the likely lethal outcome in a short time.
Distinguish between fulminant and delayed forms. When poison enters the body in large quantities, death can occur almost instantly. The affected person immediately loses consciousness, breathing becomes frequent and superficial, the pulse quickens, arrhythmic, convulsions occur. The convulsive period is short, breathing stops and death occurs. With a delayed form, the development of poisoning can be extended in time and proceed in various ways.
Mild degree of poisoning characterized mainly by subjective disorders: irritation of the upper respiratory tract, conjunctiva of the eyes, an unpleasant burning-bitter taste in the mouth, the smell of bitter almonds is felt, weakness, dizziness appear. A little later, there is a feeling of numbness of the oral mucosa, salivation and nausea. At the slightest physical effort, shortness of breath and severe muscle weakness, tinnitus, difficulty in speech appear, and vomiting is possible. After the cessation of the action of the poison, all unpleasant sensations subside. However, headaches, muscle weakness, nausea, and a feeling of general weakness may remain for several days. With a mild degree of intoxication, complete recovery occurs.
With intoxication medium degree in the beginning, the subjective disorders described above are noted, and then a state of excitement arises, a feeling of fear of death appears. The mucous membranes and skin become scarlet, the pulse is slow and tense, blood pressure rises, breathing becomes shallow, short-term clonic convulsions may occur. With timely assistance and removal from the contaminated atmosphere, the poisoned quickly regains consciousness. In the next 3-6 days, weakness, malaise, general weakness, headache, discomfort in the region of the heart, tachycardia, restless sleep.
In the clinical picture severe intoxication There are four stages: initial, dyspnoetic, convulsive and paralytic. initial stage characterized mainly by subjective sensations, as described above in the description lung poisoning degree. It is short-lived and moves on to the next one. For the dyspnoetic stage, some signs of oxygen starvation of the tissue type are typical: the scarlet color of the mucous membranes and skin, gradually increasing weakness, general anxiety, and discomfort in the region of the heart. The poisoned person develops a feeling of fear of death, pupils dilate, the pulse slows down, breathing becomes frequent and deep. In the convulsive stage, the condition of the affected person deteriorates sharply. Consciousness is lost, the corneal reflex is sluggish, the pupils do not react to light. Exophthalmos appears, breathing becomes arrhythmic, rare, blood pressure rises, the pulse rate decreases. There are widespread clonic-tonic convulsions. The scarlet color of the skin and mucous membranes is preserved. The duration of this stage can vary from several minutes to several hours. With further deterioration of the condition of the affected person, the paralytic stage develops. Convulsions by this time stop, however, the patient has a deep coma with a complete loss of sensitivity and reflexes, muscle adynamia, involuntary urination and defecation are possible. Breathing is rare, irregular. Then comes a complete cessation of breathing, the pulse quickens, becomes arrhythmic, blood pressure drops and after a few minutes cardiac activity stops.
Consequences and complications characteristic of severe intoxication. Within a few weeks after the injury, persistent and profound changes in the neuropsychic sphere may persist. As a rule, asthenic syndrome persists for 10-15 days. Patients complain of increased fatigue, decreased performance, headache, bad dream. There may be violations of motor coordination, persistent disorders of the cerebellar nature, paresis and paralysis of various muscle groups, difficulty in speech, mental disorders. From co-
matic complications in the first place is pneumonia. Its occurrence is facilitated by aspiration of mucus, vomit, prolonged stay of patients in a supine position. Changes are also observed in the cardiovascular system. Within 1-2 weeks, discomfort in the region of the heart, single extrasystoles, tachycardia, lability of the pulse and blood pressure indicators are noted, ECG changes (signs of coronary insufficiency) are traced.
DIAGNOSTICS OF POISONING WITH PRUSIAN ACID
The diagnosis of hydrocyanic acid damage is based on the following signs: the sudden onset of symptoms of the lesion, the sequence of development and the transience of the clinical picture, the smell of bitter almonds in the exhaled air, the scarlet color of the skin and mucous membranes, wide pupils and exophthalmos.
TREATMENT OF POISONING WITH PRUSIAN ACID
The effect of helping those poisoned by cyanide depends on the speed of application of antidotes and agents that normalize the functions of vital organs and systems.
Methemoglobin-forming substances, substances containing sulfur and carbohydrates have antidote properties. Methemoglobin-forming agents include anticyan, amyl nitrite, sodium nitrite, methylene blue. They oxidize the iron in hemoglobin, turning it into methemoglobin. Methemoglobin containing ferric iron is able to compete with cytochrome oxidase for cyanide. It should be borne in mind that methemoglobin is not able to bind with oxygen, therefore, it is necessary to use strictly defined doses of these agents, since hemic hypoxia develops when hemoglobin is inactivated by more than 25-30%. Methemoglobin binds primarily to cyanide dissolved in the blood. With a decrease in the concentration of cyanide in the blood, conditions are created for the restoration of cytochrome oxidase activity and the normalization of tissue respiration. This is due to the reverse flow of cyanide from the tissues into the blood - towards a lower concentration. The formed cyan-methemoglobin complex is a fragile compound. After 1-1.5 hours, this complex begins to gradually decompose with the formation of hemoglobin and cyanide. Therefore, a relapse of intoxication is possible. However, the dissociation process is extended in time, which makes it possible to neutralize the poison with other antidotes.
The standard antidote from the group of methemoglobin-forming agents is anticyan.
In case of hydrocyanic acid poisoning, the first injection of anticyan in the form of a 20% solution is made in a volume of 1.0 ml intramuscularly or 0.75 ml intravenously. When administered intravenously, the drug is diluted in 10 ml of 25-40% glucose solution or saline, the rate of administration is 3 ml per minute. If necessary after 30 min. the antidote can be re-introduced at a dose of 1.0 ml, but only intramuscularly. After another 30-40 min. a third administration at the same dose can be given if indicated.
Sodium nitrite is a powerful methemoglobin former. Aqueous solutions of the drug are prepared ex tempore, as they are unstable during storage. When assisting poisoned sodium nitrite is administered intravenously slowly in the form of a 1-2% solution in a volume of 10-20 ml.
Amyl nitrite, propyl nitrite has a methemoglobin-forming effect. Methylene blue has a partial methemoglobin-forming effect.
Substances containing sulfur. When substances containing sulfur interact with cyanide, non-toxic rhodanide compounds are formed. Sodium thiosulfate turned out to be the most effective sulfur donator. It is administered intravenously in 20-50 ml of a 30% solution. It reliably neutralizes the OV. The disadvantage is the relatively slow action.
The next group of antidotes has the property of converting cyan into non-toxic cyanohydrins. This property is observed in carbohydrates. Glucose has a pronounced antitoxic effect, which is recommended to be administered at a dose of 30-50 ml of a 25% solution. In addition, glucose has a beneficial effect on respiration, heart function and increases diuresis.
An antidote effect is observed when cobalt salts are used, which, when interacting with cyanides, lead to the formation of non-toxic cyano-cobalt compounds.
The effect of antidotes is enhanced when they are used against the background of oxygen barotherapy. It has been shown that pressurized oxygen contributes to more quick recovery cytochrome oxidase activity.
There is information about favorable therapeutic effect unithiol, which, being not sulfur donators, activates the enzyme rhodonase, and thus accelerates the detoxification process. Therefore, it is advisable to introduce unithiol along with sulfur donors.
Antidote therapy for lesions with hydrocyanic acid, as a rule, is carried out in combination: first, methemoglobin formers are used, then sulfur donors and substances that promote the formation of cyanohydrins.
In addition to the use of antidotes, it is necessary to carry out all the general principles of treating poisoned people (removal of non-absorbed and absorbed poison, prevention of further entry of poison into the organs - by the method of forced removal, symptomatic therapy, resuscitation).
STAGE TREATMENT
Poisoning develops quickly, so medical care is in the nature of an emergency.
First aid in the outbreak includes putting on a gas mask on the poisoned person. Then evacuation is carried out outside the outbreak. Affected in an unconscious state and the convulsive stage of intoxication need to be evacuated lying down.
First aid is carried out outside the hearth, which allows you to remove the gas mask. Antician is introduced - 1 ml intramuscularly, if necessary, cordiamin, mechanical ventilation.
First aid. The anticant is reintroduced. If not assigned at the stage first aid, it is desirable to carry out the first injection intravenously with 10 ml of a 25-40% glucose solution. Subsequently, 20-50 ml of a 30% solution of sodium thiosulfate is injected intravenously. According to the indications, 2 ml of a solution of etimizole and cordiamine are used intramuscularly, mechanical ventilation.
Further evacuation is carried out only after the elimination of convulsions and normalization of breathing. Along the way, it is necessary to provide assistance in case of recurrence of intoxication.
Qualified therapeutic assistance consists in carrying out, first of all, urgent measures: repeated administration of antidotes (anticyan, sodium thiosulfate, glucose), injections of cordiamine, etimizol, mechanical ventilation (hardware method). Delayed measures of qualified therapeutic care include the introduction of antibiotics, sulfonamides, desensitizing agents, vitamins.
Those affected in a coma and convulsive state are not transportable. The evacuation of the seriously injured is carried out in the VPTG, in the presence of neurological disorders - in the VPNG, those who have undergone mild intoxication remain in the medical hospital (OMO).
Specialized assistance is provided in the corresponding therapeutic hospitals (VPTG, VPNG) in full. At the end of treatment, the convalescents are transferred to the HPRL, in the presence of persistent changes in the nervous, cardiovascular, respiratory systems, patients are subject to referral to the IVC.
Potassium cyanide is widely used in the extraction of precious metals such as gold and silver from ore. For this, a technique such as cyanidation is used. In addition, potassium cyanide is used in the galvanization of products. What about domestic use, then cyanide is used in reagents, for photographs, and the poison is also part of various stains for the destruction of insects. Cyanide does not occur in its pure form in nature. However, some compounds with cyanides are present in the stones: peaches, cherries, almonds. Our online store offers potassium cyanide to buy, at the most affordable prices. However, first, let's figure out what this substance is.
Did you know?
A few words about interesting facts associated with the discovery and use of potassium cyanide. There are several controversial versions about whether it really smells like almonds, but this does not interest us at the moment. Back in 1845, the chemist Robert Bunsen conducted experiments in his laboratory and, as the author of the spectral analysis method, recorded the production of potassium cyanide. It was this scientist who developed the method of its industrial production. It is today the sale and synthesis of such a substance is strictly controlled. It is now possible to produce CK under isolated laboratory conditions. But in late XIX, the question "where to buy cyanide?", in general, was simply solved. That is why potassium cyanide was especially popular among attackers. Detective lovers may remember how in Agatha Christie's story " Vespiary”, this substance was acquired with a criminal intent, hiding behind the intention to poison wasps. And only thanks to Poirot, the criminals did not succeed.
Potassium cyanide in entomology
As many years ago, today the use of potassium cyanide to control insects is also allowed. So there are special stains in which a small amount of cyanide is placed. A few crystals of poison are placed on the bottom of the container and poured with liquid gypsum on top. Thus, it happens chemical reaction, the result of which is the release of hydrogen cyanide. By inhaling the poison, the insects die, and the stain acts in this way. A properly charged trap can be "active" for a year, and sometimes even longer. Among chemists, potassium cyanide is called the "king of poisons". Where can you buy cyanide? We will talk about this a little later. To begin with, let's "get acquainted" with him, clarify the goal-setting of such an acquisition.
Cyanide - features
Potassium cyanide is a salt of hydrocyanic acid. Externally, cyanide resembles ordinary sugar. It is also important to note that cyanide is one of the most potent organic poisons. It is noteworthy that of all the toxic substances, it is potassium cyanide that has the most notoriety. In almost all detective novels, cyanide is used by attackers to eliminate their enemies and enemies. This is primarily due to the fact that at the end of the nineteenth and at the beginning of the twentieth century, anyone could buy potassium cyanide at any pharmacy. In addition, it should be noted that this substance is easily soluble in ordinary water. If a person has a good sense of smell, he can hear a specific smell, similar to almonds.
Looking into our online store, everyone can buy potassium cyanide, the price of which will satisfy even the most demanding buyer. This became possible due to the fact that the managers have established close cooperation with the producers of potassium cyanide. The declared cost does not include interest for the services of intermediaries. If you are interested in such questions as, what is potassium cyanide, where can I get this substance? Then this article will undoubtedly arouse genuine interest in you.
The impact of potassium cyanide on the human body
Potassium cyanide can enter the human body through the skin, especially if there are any injuries on the body. Also, the powder or vapors of the cyanide solution can be inhaled. For humans, the lethal dosage is 1.7 mg per kilogram of body weight. Therefore, when using the substance, it is very important to observe all precautions.
An interesting fact remains that the effect of cyanide on the human body is significantly weakened when combined with glucose. That is why specialists who, by virtue of their professional activity forced to work with cyanide, hold a small piece of sugar behind the cheek. Thus, they neutralize poisonous toxins that have entered the bloodstream. A vivid confirmation of this can be historical fact like the poisoning of Rasputin. Indeed, in order to send Gregory "to the next world", cyanide was sprinkled into sweet flour products. Even despite the fact that the dose of the poisonous substance was too high, the sugar did its job, and it was not possible to immediately finish off Rasputin.
There is also a chronic form of poisoning, when a person works in a factory where this poisonous substance is used, the poison in the body accumulates gradually. Therefore, it is very important to pay attention to such signs of poisoning: sleep disturbance, frequent headaches, a feeling of squeezing in the heart, weight loss.
Main symptoms of cyanide poisoning
The impact of such a poisonous substance as cyanide on the human body manifests itself almost instantly. Symptoms of poisoning directly depend on the dose received, as well as on how sensitive the human body is to this poison.
- Large salivation
- Dizziness
- Feeling of numbness in the mouth and throat
- Compression in the chest area
- Perspiration and bitterness in the mouth
If a lethal dose of cyanide has entered the human body, symptoms such as fainting, convulsions, uncontrolled urination, biting of the tongue can be observed. The cause of death is respiratory and cardiac arrest. Therefore, before you get potassium cyanide and start working with this potent poisonous substance, you need to weigh the pros and cons well.
Chronic cyanide poisoning
Very often, when working with substances that contain cyanide, signs of chronic poisoning may occur. In this case, the picture of the pathological condition will be blurred and stretched in time. A person may experience the following symptoms:
- Constant headache
- Dizziness
- Pain and discomfort in the region of the heart
- Increased sweating
- Frequent urge to urinate
In addition, signs of irritability will melt away, thyroid pathologies become aggravated, the skin begins to peel off, itching and other unpleasant symptoms may occur. That is why, when working with chemical elements it is important to use personal protective equipment.
Where can you buy potassium cyanide?
Many people, in an attempt to find potassium cyanide, turn to workers who work in gold or silver refineries. Some people try to bribe the medical staff working in medical institutions. It should be noted that in most cases such attempts remain unsuccessful. This is due to the fact that this chemical drug quite dangerous and accountable, so it is unlikely that anyone will risk their workplace. However, thanks to our virtual resource, there is no need to wonder where to find potassium cyanide. Everything is quite simple, the user enters the site and leaves a request for a purchase.
In addition to all of the above, I would like to note that it will not work to stock up on potassium cyanide for the future. The thing is that hydrocyanic acid corresponding to cyanide nion is a very weak substance, so it is easily displaced by other acids. Thus, cyanide can easily become completely harmless. To do this, you can conduct a simple experiment. You just need to leave the cyanide for a period of time on outdoors, under influence carbon dioxide and moisture, it will lose all its poisonous abilities.
Some people who need potassium cyanide try to make this poisonous substance at home. This is possible if a person is familiar with chemistry and has all the necessary reagents at hand. However, experts do not advise risking their health and life, because unlucky chemists can easily become poisoned by toxic fumes. Therefore, the most right decision will be potassium cyanide to buy on our marketplace.
For what purposes can cyanide be used?
Decades ago, this poison was isolated by processing certain plants in the laboratory. Today it is produced synthetically. Where is potassium cyanide used? In agriculture and industry, the use of cyanides is widely known. Such a “popularity” of the remedy allows one to close one’s eyes to its slight toxicity. So for the production of plastics, cyanide compounds are widely used.
We all love to take pictures or be photographed, but in reactives, cyanide, already known to us, is used to develop photos. If the poison is presented in the form of a gas, then it is rational to use it to treat agricultural facilities where grain is stored in order to protect against rodents. But history knows cases of inhumane use of a chemical substance. So in concentration camps, the Nazis used Zyklon-B gas, which included poison.
Legal sale of cyanide substances
In our online store you can find potassium cyanide at an affordable price. All products of the service are certified, which means that Chemical substance meets its specifications. In addition, choosing a convenient payment method for you, you can arrange delivery of the purchase. Why buy from us?
- Affordable prices
- Individual approach
- The product is always in stock
- Only quality products
How to help with potassium cyanide poisoning?
If you have neglected the rules of personal protection or violated the storage conditions of the substance, you need to know how to help a person with cyanide poisoning. First you need to provide access fresh air and call an ambulance. If the poison got through the skin, clothes soaked with it, it is worth removing it so that the substance does not enter the body further. When ingesting poisonous contents inside, you need to rinse the stomach. When providing first medical aid, you need to be as collected and accurate as possible. Cardiac massage, if necessary, can be done. But artificial respiration is not recommended, because the one who resuscitates can be poisoned by poison vapors.
In a hospital setting, doctors prescribe complex treatment. Sugars are used as the first antidote. This may be a glucose solution that is administered intravenously. Sugar helps to deactivate the poison, turning it into harmless metabolites. The second antidote may be sodium thiosulfate. Its function is reduced to the conversion of cyanides into thiocyanates, which do not pose a danger. human body. And you can not do without drugs that lead to the formation of cyanmethemoglobin, it can be nitroglycerin, amyl nitrite, methylene blue.
Safe online shopping
Using our service, you can be sure that having paid money for the goods, you will be satisfied with the result. There are no hidden fees or unpleasant surprises. In addition, the customer base of the service is not disclosed, but if you wish, you can always leave your feedback about the purchase.
Here you can choose potassium cyanide, the price of which will be affordable for you. Delivery throughout the country. We are confident that you need a chemical for useful things, trusting our customers. Before buying cyanide, take care of protective equipment and storage conditions.
Shop benefits:
- Prompt order processing
- For regular customers- discounts
- Wholesale and retail trade
Take care of yourself and your health! Remember, even a medicine in violation of the dosage can be poison! Buy from trusted stores!