Types of heat treatment of metals. Heat treatment of alloys. Types of heat treatment

Read also
Heat treatment of metals
But for further exploitation parts and components of equipment often require completely different characteristics - strength, hardness, rigidity, etc. It is for these purposes that the heat treatment of metals is intended.
The essence of heat treatment processes
tasks various technologies heat treatment is:
- Ensuring the most favorable microstructure of steels and alloys;
- Obtaining the desired level of hardness: either in a thin surface (or subsurface) zone, or throughout cross section blanks;
- Correction chemical composition in grains of macrostructures various alloys.
In the first case, it is necessary to ensure the maximum degree of homogeneity of the properties of metals, which is important, for example, for subsequent mechanical or, especially, deforming processing. As a result, the conditions for the shape change of the workpiece in all three coordinate axes are the same, and the marriage of the final part is excluded.
In addition, the alignment of the micro and macrostructure for metal forming processes is necessary in order to increase the degree of deformation of semi-finished products, ultimately bringing the shape of the workpiece closer to the shape of the finished product. Moreover, for the least number of transitions, and using the minimum equipment effort required for this.
The change in hardness (as a result of heat treatment) is intended to improve the performance of parts. Since the operating conditions can be very different, the complex of physical and mechanical properties is selected strictly individually: there are no universal heat treatment processes for alloys with different compositions.
A change in the chemical composition in the grains of the microstructure, due to the formation of new compounds, in most cases not only raises the hardness indicators, but also increases the wear resistance of parts that must be operated at increased friction, temperature, or increased against normal specific loads.
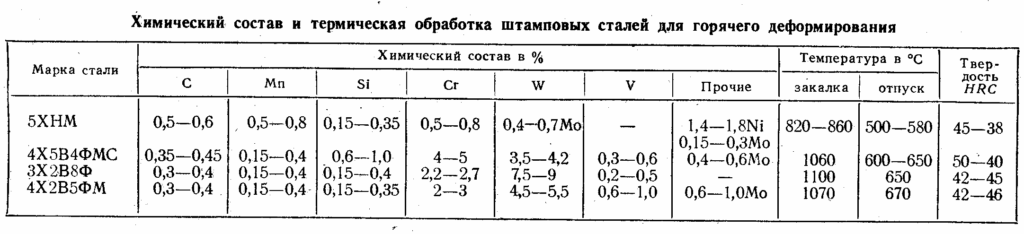
hardening-tempering
The first group of heat treatment technologies for various alloys, including steel, includes annealing and tempering. In the second - hardening, normalization, improvement, aging, cold treatment. In the third - all types of thermochemical processing.
Annealing
The essence of the processes occurring in the structure of most alloys subjected to annealing is to provide the most balanced structure of the workpiece, in which there are either no internal stresses, or their level is sufficiently low, and therefore does not affect the subsequent machinability of metals/alloys.

The initial structure of almost all alloys and steels is a rather large grains, between which there are inclusions and impurities, mainly sulfur and phosphorus. This increases the brittleness of the metal, which may be important when forming products of complex configuration from an ingot (or wire rod). Therefore, it is necessary to reduce the grain size and give it the optimal shape of an ellipsoid, in which the mechanical properties will be approximately the same along all three coordinate axes.
For this purpose, the original workpiece must be heated to a temperature of 50 ... 70 0 C above the temperature of the beginning of the austenite transformation. Its result is the formation of small and well-oriented austenite grains between the grains of the main structural components of steel - ferrite and cementite. Austenite is formed from perlite, a structure that has the largest grains, which contributes to the increased fragility of any ingot. Austenitic transformation for most alloys proceeds quite slowly, so annealing is a lengthy procedure that should last at least an hour.

Second important task annealing - remove the internal stresses that are formed in the workpiece during its processing by pressure in the cold state. The fact is that any deformation is accompanied by grain crushing of the original structure of steels and alloys. As a result, there are more grains, the deformation resistance increases, which not only requires an increased deformation force, but also causes the destruction of the semi-finished product, the degree of deformation of which has exceeded the critical indicator for this metal.
Accordingly, to implement the first task, high-temperature annealing technology is used (for steels, depending on the carbon content, it ranges from 550 ... 750 0 С), and in the second - low-temperature annealing (180 ... 220 0 С).
 High temperature annealing methods
High temperature annealing methods Heating occurs slowly, followed by keeping the product at a given temperature, followed by slow cooling. For alloy steels and alloys, such cooling is carried out at a particularly low rate, in the furnace itself, where the annealing took place.
Vacation
Tempering according to the technology resembles annealing, but is carried out not with a workpiece, but with a finished product, and therefore pursues other tasks - to relieve internal stresses after heat treatment, which was carried out to increase the hardness of the part.

Self process heat treatment leave is not. Unlike annealing, tempering is sometimes carried out in several steps: in most cases, this applies to products for the production of which were used different kinds high alloy steel.
hardening
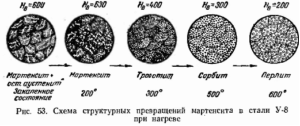
Hardening consists in the rapid heating of the workpiece to the temperature of the end of the austenitic transformation (900 ... 1100 0 C - for low-carbon steels, 750 ... 850 0 C - for high-carbon steels) and subsequent rapid cooling in special quenching media. As the latter, water is used (for low-responsibility products) or oil.
Hardening modes are the most diverse. The main factor determining the effectiveness of hardening is the intensity of formation of martensite in the structure - a high-temperature component that gives the metal or alloy increased hardness.
The conditions for the formation of martensite are determined by the following circumstances:
Accordingly, for each grade of steel or alloy, individual hardening modes have been developed, which differ:

Hardening of steels and alloys with a complex composition, including several alloying elements (in particular, cobalt, molybdenum), is carried out with particular care. These metals in the process form intermetallic compounds along the grain boundaries of the main structure, which significantly increase the hardness and strength of steels (in particular, tool steels). The shape and concentration of intermetallic compounds depend only on the accuracy of the hardening technology.
The types of hardening are determined by the equipment on which it is performed. For example, for products such as gears, shafts, column guides, where required optimal combination high surface hardness and relatively tough core, surface hardening by high frequency currents is used.
To do this, the product is placed in an induction coil, through which a high-frequency (up to 15,000 ... 25,000 Hz) current is passed. Penetrating to a limited depth, this current contributes to an increase in the surface strength of steels or alloys. As a result, the fatigue strength of parts that operate under cyclically varying tensile-compressive stresses increases markedly.
A more intense change in the hardness of the part surface can be obtained by using high-energy heat sources for hardening - a spark or arc discharge. Discharges must be excited in liquid medium, where the workpiece or part is placed.
After quenching, in the vast majority of cases, tempering is necessary, otherwise the excessive final hardness of the part becomes the cause of increased brittleness under shock loads.
Improvement and normalization
As types of heat treatment, these processes are similar to annealing, although they are intended for other purposes - to increase the operational durability of critical machine parts and tools.
During normalization, the part is subjected to slow heating, maintained at a given temperature, after which it is necessarily cooled together with the furnace. As a result, the structure of the part becomes more balanced, and the level internal stresses goes down.
A significant difference is the composition of the atmosphere, the furnace in which these heat treatment operations are performed. It must be non-oxidizing, since intense oxide formation on the surface of the product not only worsens its presentation, but also changes its dimensions. Carbon burnout, which also accompanies heat treatment in a conventional furnace, worsens the chemical composition of the steel and reduces its strength.
Reducing the access of oxygen to the surface of the part during normalization is performed in several ways:
- Heating with a planned lack of oxygen. In this case, the stability gas burners heat treatment furnaces are compensated by increasing the rate of air supply to the combustion zone;
- Heat treatment in a protective gas environment. For critical parts, lithium vapor, argon or other noble gases are used, in other cases - carbon dioxide;
- By applying protective coatings to the surface of the product to be normalized.
After normalization, the part is cooled in still air, preventing it from blowing: this can cause an inhomogeneous, “spotty” microstructure of the product.

Improvement is a heat treatment operation, as a result of which the machinability of steels and alloys is increased, and the level of residual stresses in them is reduced. This is accompanied by some decrease in hardness.
The martensitic component in the structure of most steels and alloys can appear not only at increased, but also at low temperature. Cold treatment technology compares favorably with traditional technologies heat treatment as follows:

The modes of cryogenic treatment depend on the grade of steels/alloys and fluctuate in the range of -60…-140 0 С. low temperatures. With a combination of heating and cooling cycles, the complete decomposition of residual austenite is achieved in 4 ... 7 hours (higher values \u200b\u200bfor high-alloy steels).
A special type of heat treatment is the processes of chemical-thermal treatment. Their task is to form carbides and nitrides in the surface microstructure - compounds that significantly increase the microhardness of parts and create residual compressive stresses in them. Such products show particularly high resistance to alternating loads.
Video: How to harden any steel grade
Metal processing thermally is a change internal structure(structure) of metal under the influence of change temperature conditions and obtaining, as a result, the necessary mechanical and physical properties metal. A huge part of heat treatment takes place at critical temperatures at which structural transformation occurs in alloys.
Therefore, heat treatment of metal is reduced by three consecutive operations and types:
- heating the metal at a certain rate to a given temperature;
- exposure of the metal for some time at this temperature;
- cooling at the rate specified by the process.
It depends on how to change the properties of a particular steel product and various types of heat treatment are used, which differ in the maximum heating temperature, holding time and cooling rate. In mechanical engineering, heat treatment has found the widest application.
Heat treatment of metal, alloy, steel
 All properties of any alloy depend on its structure. The main way that allows you to change this structure is heat treatment. Its foundations were developed by D.K. Chernov, and later his work was supported by A.A. Bochvar, G.V. Kurdyumov, A.P.
All properties of any alloy depend on its structure. The main way that allows you to change this structure is heat treatment. Its foundations were developed by D.K. Chernov, and later his work was supported by A.A. Bochvar, G.V. Kurdyumov, A.P.
Heat treatment of metal and alloy is a sequence of operations such as: heating, holding and cooling, which are performed in a certain sequence and under a certain mode, in order to change the internal structure of the alloy and obtain the desired properties, while the chemical composition of the metal does not change.
What is the heat treatment of metal and alloy?
- in annealing
- hardening
- Vacation
- Normalization
Annealing. This is the heating of the metal to a high temperature, and then there is a slow cooling. Annealing happens different kind- it all depends on temperature regime heating and cooling rate.
hardening. Heat treatment of steel, alloys, metal, which is based on the recrystallization of steel when heated above critical temperature. After holding the steel at this temperature, a very rapid cooling follows. Such steel has a non-equilibrium structure and therefore, after hardening, tempering follows.
Vacation. It is carried out after hardening in order to reduce or remove residual stress in steel and alloys, increase toughness, reduce the hardness and brittleness of the metal.
Normalization. It is similar to annealing, the only difference is that the normalization of the metal occurs in air, and annealing takes place in a furnace.
Workpiece heating
 This operation is very responsible. From her proper conduct depends, firstly, on the quality of the product, and secondly, on labor productivity. It is necessary to know that when heated, the metal changes the structure, properties and all characteristics of the surface layer. Since when steel or alloy interacts with air, iron is oxidized and scale is formed on the surface. The thickness of the scale depends on the chemical composition of the metal, the temperature and time of its heating.
This operation is very responsible. From her proper conduct depends, firstly, on the quality of the product, and secondly, on labor productivity. It is necessary to know that when heated, the metal changes the structure, properties and all characteristics of the surface layer. Since when steel or alloy interacts with air, iron is oxidized and scale is formed on the surface. The thickness of the scale depends on the chemical composition of the metal, the temperature and time of its heating.
Steel begins to oxidize rapidly when heated above 900 degrees, then the oxidizability doubles - when heated to 1000 degrees C, and at a temperature of 1200 degrees C - 5 times.
What is the oxidation of different steels?
Chrome nickel steel- it is called heat-resistant because it is practically not amenable to oxidation.
Alloy steel- it forms a dense, but thin layer scale, which protects against further oxidation and prevents cracking during forging.
Carbon steel- it loses about 2–4 mm of carbon from the surface when heated. This is very bad for the metal, as it loses its strength, hardness and steel deteriorates in hardening. And especially very detrimental is decarburization for forging small parts, followed by hardening. To avoid cracks in high-alloy and high-carbon steel, they must be heated slowly.
Be sure to refer to the "iron-carbon" diagram, where the temperature for the start and end of forging is determined. This must be done so that the metal, when heated, does not acquire a coarse-grained structure and its plasticity does not decrease.
But overheating of the workpiece can be corrected by heat treatment, but this requires additional energy and time. If the metal is heated to higher temperature, then this will lead to overburning, which will come to the point that the bond between the grains is broken in the metal and it will completely collapse during forging.
Burnout
 it the most incorrigible marriage. When heating a metal or alloy, it is imperative to monitor the temperature, time and end of heating. Dross grows if the heating time is extended, and cracks may appear with rapid or intense heating.
it the most incorrigible marriage. When heating a metal or alloy, it is imperative to monitor the temperature, time and end of heating. Dross grows if the heating time is extended, and cracks may appear with rapid or intense heating.
The overheating of the alloy occurs due to oxygen diffusion at the grain boundaries, where oxides are immediately formed, which separate the grains at a high temperature of the alloy and, at the same time, the strength immediately drops sharply. And plasticity at this time comes to zero. This marriage is immediately sent for melting down.
What is the heat treatment of metal and alloys
Heat treatment subdivided into:
- thermal;
- thermomechanical;
- chemothermic
AT thermal processing includes the main types - annealing of the 1st kind, annealing of the 2nd kind, hardening and tempering. Normalization is not applied to all types of steel, it all depends on its degree of alloying.
All types of heat treatment have different heating temperatures, the duration of holding at this temperature and the cooling rate after the end of holding.
The first kind of annealing is diffusion annealing, stress relief annealing.
The second kind of annealing is subdivided into incomplete, complete, isothermal annealing, spheroidization, and normalization.
Hardening is used to ensure that products are hard, strong and wear resistant.
Chemical thermal treatment
 This is such a heat treatment of steel, which is coupled with the saturation of the surface of the product - carbon, nitrogen, aluminum, silicon, chromium, etc., which form substitutional solid solutions with iron. They are longer and more energy-intensive than steel saturated with iron and carbon, which forms interstitial solid solutions with iron.
This is such a heat treatment of steel, which is coupled with the saturation of the surface of the product - carbon, nitrogen, aluminum, silicon, chromium, etc., which form substitutional solid solutions with iron. They are longer and more energy-intensive than steel saturated with iron and carbon, which forms interstitial solid solutions with iron.
Chemical-thermal treatment, when creating favorable residual compressive stresses on the surface of products, increases the durability and reliability of the product. She also raises corrosion resistance, hardness.
Such processing is intended to change the composition of steel in a certain layer. These methods include:
- cementation - with this method upper layer steel is enriched with carbon. In this case, products with combined properties are obtained - a soft core and a hard core. surface layer;
- nitriding - e enrichment of the surface layer with nitrogen to increase the corrosion resistance and fatigue strength of the product;
- boriding - it's saturation surface layers steel with boron, with this method, the wear resistance of the product increases, especially during friction and dry sliding. In addition, when boriding, cold seizure or welding of parts is excluded. Details after boriding are made very resistant to acid and alkali;
- aluminizing - this is the saturation of steel with aluminum. This is done in order to give steel resistance to aggressive gases - sulfuric anhydride, hydrogen sulfide;
- chrome plating - saturation of the surface layer of steel with chromium. Chromium plating of mild steels has almost no effect on their strength characteristics. Chrome plating of steel with a higher chromium content is called hard chromium plating, as chromium carbide is formed on the surface of the parts, which has:
- high hardness
- scale resistance
- corrosion resistance
- increased wear resistance
 This is a hardening heat treatment of metal and alloys at cryogenic, very low temperatures - below -153 degrees C. Previously, such heat treatment was called "cold treatment" or "heat treatment of metal at a temperature below zero". But these names did not quite reflect the whole essence of cryogenic processing.
This is a hardening heat treatment of metal and alloys at cryogenic, very low temperatures - below -153 degrees C. Previously, such heat treatment was called "cold treatment" or "heat treatment of metal at a temperature below zero". But these names did not quite reflect the whole essence of cryogenic processing.
Its essence is as follows: the parts to be processed are placed in a cryogenic processor, where they are slowly cooled, and then the parts are kept at a temperature of -196 degrees C for a certain time. Then they gradually return back to room temperature. When this process takes place, structural changes occur in the metal. Due to this, wear resistance, cyclic strength, corrosion and erosion resistance are increased.
The main properties obtained during processing, such as cold cooling, are retained for the entire service life of the workpiece and therefore do not require re-treatment.
Of course, cryogenic technology will not replace the methods of thermal hardening, but when processed with cold, it will give the material new properties.
Ultra-low temperature treated tools allow businesses to cut costs because:
- increases the wear resistance of tools, parts and mechanisms;
- the number of marriages decreases;
- the cost of repair and replacement of technological equipment and tools is reduced.
It was Soviet scientists who made it possible to fully evaluate the effect of cold treatment on metal and alloy and laid the foundation for the use of this method.
AT given time the method of cryogenic processing of products is widely used in all industries.
Mechanical engineering and metalworking:
- increases the resource of equipment and tools up to 300%;
- increases the wear resistance of the material;
- increases cyclic strength;
- increases corrosion and erosion resistance;
- relieves residual stress.
Special equipment and transport:
- increases the resource of brake discs by 250%;
- improves the efficiency of the brake system;
- increases the cyclic strength of suspension springs and other elastic elements by 125%;
- increases the resource and power of the engine;
- reduces vehicle operating costs.
Defense industry:
- increases barrel survivability up to 200%;
- reduces the effect of barrel heating on shooting results;
- increases the resource of nodes and mechanisms.
Mining and manufacturing industry:
- increases the durability of the rock cutting tool up to 200%;
- reduces abrasive wear of components and mechanisms;
- increases the corrosion and erosion resistance of equipment;
- increases the resource of industrial and mining equipment.
Audio equipment and musical instruments:
- reduces signal distortion in conductors;
- improves musical activity, clarity and transparency of sound;
- expands the sound range of musical instruments.
Cryogenic processing is used in almost all industries where it is necessary to increase the resource, increase strength and wear resistance, as well as increase productivity.
 Reliability and durability metal structures, equipment, pipelines depends on the quality of manufacturing units, parts, elements of which they consist. During operation, they are subjected to static, dynamic and cyclic loads and the influence of aggressive environments. They have to work at low and high temperatures and is under conditions of rapid wear.
Reliability and durability metal structures, equipment, pipelines depends on the quality of manufacturing units, parts, elements of which they consist. During operation, they are subjected to static, dynamic and cyclic loads and the influence of aggressive environments. They have to work at low and high temperatures and is under conditions of rapid wear.
And therefore, the operation of any metal products directly depends on the wear resistance, strength, thermal and corrosion resistance of the elements of which they are composed.
In order to improve all these characteristics, it is necessary to choose the right material for parts, improve their design, eliminate assembly inaccuracies, and improve hot and cold working methods.
Such high requirements are seldom met by materials in the delivered condition. The main part of delivered structural elements needs stabilization operational properties so they don't change over time. And to enhance the mechanical and physiochemical properties metal materials, apply heat treatment. This is a sequence of operations of heating, holding and cooling metals and alloys.
It is carried out to change the structure and properties of metals and alloys in the direction that has been set. Heat treatment is used to change the structure of the phase composition and redistribution of components, the size and shape of crystalline grains, types of defects, their number and distribution. And all this makes it quite easy to obtain the required property of the material.
It must be remembered that the properties of metal and alloys depend not only on the structure, but also on the chemical composition that is formed during the metallurgical and foundry process.
The task of heat treatment is the elimination of internal stress in the metal and alloy, the improvement of mechanical and operational properties, and more.
subjected to heat treatment steel, cast iron, non-ferrous alloy.
You need to know that materials with the same chemical composition during different modes heat treatment, you can get several completely different structures that will have completely different properties. By improving the mechanical properties through heat treatment, alloys with a higher simple composition. Permissible stresses, reducing the mass of parts and mechanisms, increasing their reliability and durability can also be achieved using heat treatment.
Topic 1.2 Thermal and chemical-thermal treatment of metals and alloys
Heat treatment is a process, the essence of which is the heating and cooling of products according to certain modes, as a result of which there is a change in the structure, phase composition, mechanical and physical properties. Various types of chemical-thermal treatment are also used, the essence of which is alloying the surface layer of products with nitrogen, carbon, or some metals (aluminum, chromium, beryllium) with subsequent heat treatment.
Depending on the purpose of heat treatment, there are various types of it, differing in heating temperature, holding time and cooling rate.
The main types of heat treatment of steel are annealing, normalizing, hardening, and tempering.
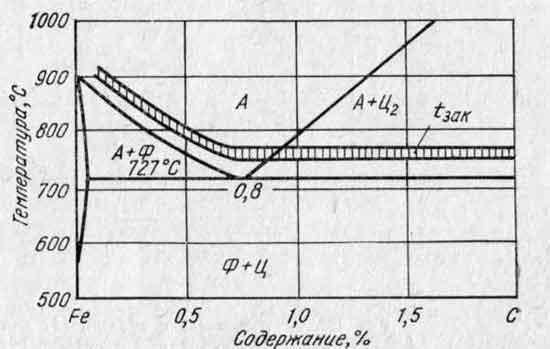
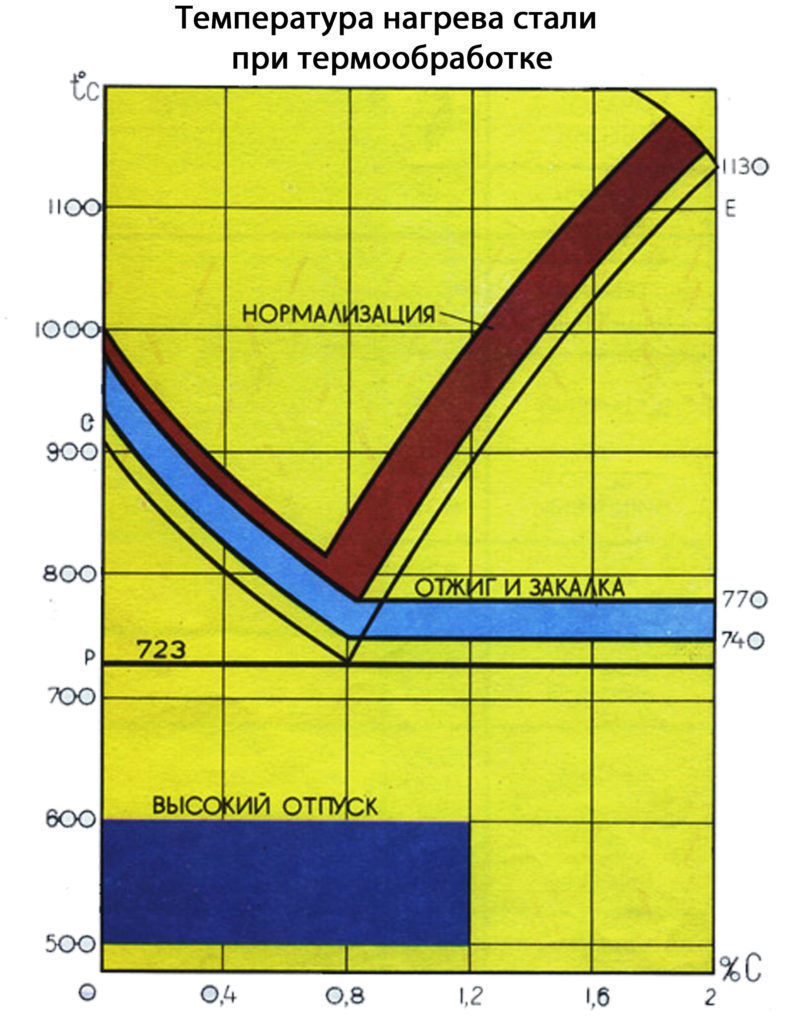
Annealing applied to reduce hardness, grain refinement, improve machinability, stress relief. For annealing, steel is heated to a certain temperature, held and cooled at low speed in a furnace with the heat source turned off.
Normalization - represents the heating of steel to a temperature above the critical point, holding at this temperature, cooling in still air at a rate greater than during annealing. Purpose - giving steel a fine-grained structure. Normalization gives a more noticeable increase in strength, but less ductility. The advantage over annealing is lower cost.
Hardening - It consists in heating the steel, holding it at a given temperature for a certain time and then abruptly cooling it. The goal is to increase hardness and strength. During hardening, internal stresses arise in the material, ductility and impact strength sharply decrease. This can lead to brittle fracture of the part during operation.
To reduce internal stresses and increase ductility and impact strength after hardening, vacation, which consists in heating hardened steel to a temperature not exceeding the lower critical point, holding at this temperature and subsequent cooling.
Depending on the temperature, there are three types of vacation: low, medium, high.
Low tempering is carried out at a temperature of 150...250°C. The hardness of steel decreases slightly. The structure formed as a result of low tempering is called mar-tensite release or tempered martensite. The cutting and measuring tool, dies for cold forming, as well as parts that must have high wear resistance.
Average vacation is made at a temperature of 350...450 °C. During medium tempering, cementite is formed not in the form of plates, but in the form of tiny grains, which contributes to an increase in the toughness of the steel. Products that must have high elasticity and a sufficient margin of viscosity (springs, springs, etc.) are subjected to medium tempering.
High tempering is carried out at a temperature of 500...650°C. As a result, the residual stresses are completely eliminated, the steel acquires good ductility and toughness at a sufficiently high strength. Such a vacation is intended for critical parts that experience shock and alternating loads during operation.
Double heat treatment, which includes hardening and subsequent high tempering, is called thermal improvement, since the whole complex of mechanical properties of steel is improved.
Chemical-thermal treatment. Chemical-thermal treatment consists in chemical and thermal exposure in order to change the chemical composition, structure and properties of the surface layer of the metal. In the process of chemical-thermal treatment, the surface saturation of the metal with the corresponding element occurs in the process of its diffusion in atomic form from a liquid, solid or gaseous medium at high temperature. The main types of chemical-thermal treatment are classified according to the names of the elements with which the surface layer is saturated. For example, saturation with carbon is called cementation, nitrogen - nitriding, chromium - chromium plating.
The widespread use of chemical-thermal treatment in various fields of technology is explained by the fact that, by increasing the hardness, wear resistance, and corrosion resistance of the surface, it increases the reliability and durability of the operation of machine parts and mechanisms.
Chemical-thermal treatment consists of:
dissociation, which occurs on the contact surface "metal - gaseous environment"and leads to the release of a diffusing element in the atomic state;
absorption - surface absorption of free atoms;
diffusion - penetration of the saturating element deep into the surface layer.
The main physical process is diffusion, the rate of which increases with increasing temperature, the diffusing element penetrates to a greater depth.
Carburizing consists in diffusion saturation of the surface layer of steel with carbon when heated in an appropriate medium - a carburizer. The carburizing process takes place at temperatures (900 ... 950 ° C), when austenite is stable, dissolving carbon in large quantities. Carburizing is carried out in solid, liquid and gas carburetors. Gas carburizing is the most productive process. The main reaction providing carburization is the dissociation of methane:
CH → 2H 2 + C at.
Carburizing is carried out on low-carbon and alloy steels containing from 0.1 to 0.35% carbon. The surface layer of steel after carburizing has a variable concentration of carbon in thickness, decreasing from the surface to the core of the part. In this regard, after slow cooling in the structure of the cement-bath layer, three zones can be distinguished (starting from the surface): hypereutectoid, eutectoid, hypoeutectoid. The total thickness of the cemented layer is 0.8...3.0 mm.
After cementation, to obtain the required properties, heat treatment is carried out - hardening with low tempering. As a result of heat treatment, the surface layer acquires a wear-resistant structure, and the low-carbon core of the part turns out to be quite viscous. Most often, gears of various mechanisms are subjected to carburizing in order to increase their durability.
Nitriding consists in diffusion saturation of the surface layer with nitrogen in the appropriate medium. Before nitriding, steels are subjected to heat treatment - hardening and high tempering.
Nitrided are alloyed steels containing chromium, molybdenum, vanadium and other elements capable of forming very hard and heat-resistant nitrides with nitrogen. Nitriding is carried out at a temperature of 500...600°C. The main reaction that occurs on the surface of the part is the ammonia dissociation reaction.
After nitriding, the steel acquires high hardness, wear resistance and corrosion resistance. In these properties, nitrided steels are superior to carburized ones. However, the nitrided layer resists shock loads worse and has a lower contact endurance. Due to the long duration of the process (up to 70 hours), the use of nitriding is economically feasible for processing critical products, such as car crankshafts, cylinder liners for internal combustion engines, various fittings, etc.
Chrome plating consists in diffusion saturation of the surface layer of steel with chromium at a temperature of 900 ... 1300 ° C in an appropriate medium. Chrome plating provides increased heat resistance of steel up to a temperature of 800 ° C, high corrosion and erosion resistance. Parts of steam power plants, steam pipeline fittings, valves, valves, branch pipes are subjected to chrome plating. To increase the resistance to corrosion, transformer steel is subjected to chromium plating. In this case, the steel additionally acquires high heat resistance.
test questions
What characterizes the crystal structure of metals?
What types of crystal lattices do you know?
What are the main defects in the crystal structure?
What is metal allotropy?
What mechanical properties characterize the strength and ductility of materials under tension?
What is an alloy state diagram and what does it allow you to establish?
What are the main types of heat treatment?
What is steel carburizing?
Section 2 SEMICONDUCTOR MATERIALS
Topic 2.1 Classification of semiconductor materials
Substances are distinguished by their ability to conduct electricity. This ability is characterized by the value of specific electrical resistance– ρ or electrical conductivity – γ. The value of γ at normal temperature is less than 10 -10 S/m for dielectrics, and more than 10 4 S/m for conductors.
Let us consider the nature of the change in the specific conductivity of solids at a temperature tending to absolute zero.
In this case, the specific conductivity of conductors increases, and many metals pass into a superconducting state, which is characterized by an infinite great value γ.
Semiconductors behave quite differently. As the temperature decreases, their specific conductivity decreases, and at T → 0 K, semiconductors do not conduct electric current at all, i.e. become dielectric. On the other hand, with increasing temperature, γ of semiconductors increases sharply.
For the appearance of free charge carriers in semiconductors, it is not necessary to bring them to thermal energy. This can be achieved in them by lighting, mechanical loads, electric field, etc. The internal structure of semiconductors has a strong influence on electrical conductivity. In addition, the introduction of even a small amount of atoms of a foreign element into a semiconductor usually drastically changes its electrical conductivity. All this opens wide opportunities to control the electrophysical properties of semiconductor materials.
So, A semiconductor is a substance whose main property is the strong dependence of its electrical conductivity on the influence of external factors.
All semiconductors can be divided into simple and complex.
Simple such a semiconductor is called, the main composition of which is formed by atoms of one chemical element (silicon, germanium, selenium, tellurium, boron, carbon, phosphorus, sulfur, arsenic, antimony, iodine, gray tin).
Structure difficult semiconductors is formed by atoms of various chemical elements. This group includes solid solutions (silicon and germanium) and chemical compounds, designated AB. In this formula, the superscripts (m and n) indicate the group number periodic system, which includes the corresponding element, and the lower ones (x and y) are the number of atoms of this element in the compound. For example, the semiconductor gallium arsenide GaAr is classified as a compound of type A III B V, and copper oxide Cu 2 O is classified as a compound of type A 2 I B VI.
Many ternary and more complex chemical compounds have semiconductor properties, such as ZnSiAs 2 . Semiconductor is whole line organic compounds: anthracene, naphthalene, phthalocyanine, etc.
A remarkable property of semiconductors is also that they allow the inverse transformation electrical energy into light, thermal or mechanical.
Semiconductors are used to amplify and generate electrical signals (transistors, diodes, integrated circuits), as primary temperature converters and sources of thermal energy (thermistors and heating elements), as primary converters and sources of optical signals (photoresistors, LEDs, lasers), for converting mechanical vibrations(strain resistors and piezoelectric sensors), etc.
Energy levels and zones
An atom consists of a nucleus and electrons moving in certain orbits.
The total energy of electrons, equal to the sum of its kinetic (orbital movement) and potential (attraction to the nucleus) energies, is called the energy state of the atom. Each allowed orbit has its own energy state, which is represented in the diagram as an energy level. Since the orbits and their energies are divided into allowed and forbidden ones, then energetic levels can also be allowedand prohibited.
Electron energy E expressed in electron volts. Electron-volt- is the energy thatelectron accelerating into electric field with differentpotentials in 1 V.
The interaction of atoms in the lattice leads to the fact that their energy levels are split into a large number of almost merging sublevels, forming energeticzones.
The energy band filled with sublevels is the wider, the closer the atoms are located and the higher the energy level. The probability of an electron "staying" in the band gap is zero.
Classification of materials by electricalproperties (band theory)
The classification of electrical materials according to their electrical properties is based on the ideas of the band theory of the electrical conductivity of solids, the essence of which is as follows.
In an isolated atom, electrons revolve around the nucleus in certain orbits. According to the Pauli principle, on each or-A bit can have at most two electrons. Each orbit corresponds to a strictly defined value of energy that an electron can have, i.e. each orbit represents a specific energy level. Under the influence of the attraction of a positively charged atomic nucleus electrons tend to occupy the levels closest to the nucleus with a minimum energy value. Therefore, the lower energy levels are filled with electrons, and the upper levels are free. An electron can jump from a lower energy level W x to another free level W 2 (Fig. 1). To do this, the electron must be given additional energy ∆ W = W 2 - W 1 . If there are no free levels in the atom, then the electron cannot change its energy, therefore it does not participate in the creation of electrical conductivity.
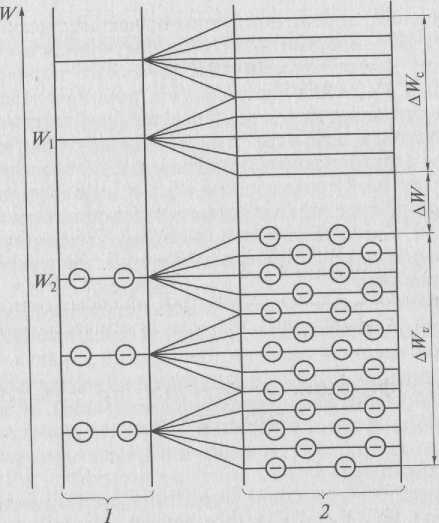
W - electron energy; W 1 - lower energy level; W 2 - free energy level; ∆ W C - conduction band (free zone); ∆ W - forbidden zone; ∆ Win, - valence band
Figure 2.1 - Diagram of energy levels of an isolated atom (1) and solid body (2).
In a crystal lattice consisting of several atoms, individual energy levels are split into sublevels that form energy zones (see Fig. 2.1). In this case, free and filled energy levels are split. The area filled with electrons is called valence. The upper level of the valence band is denoted W in . The free zone is called the zone conductivity. The lower level of the conduction band (free band) is denoted W a . The gap between the valence band and the conduction band is called prohibited zone ∆ W. The value of the band gap significantly affects the properties of materials.
If a ∆ W is equal to or close to zero, then the electrons can go to free levels due to their own thermal energy and increase the conductivity of the substance. Substances with such a structure of energy bands are referred to as conductors. Conductor materials are used to conduct electric current. Typically, conductors include substances with electrical resistivity p< 10 Ом*м. Типичными проводниками явля-ются металлы.
If the band gap exceeds a few electron volts, then significant energy is required to transfer electrons from the valence band to the conduction band. Such substances are referred to as dielectrics. Dielectrics have a high electrical resistivity and have the ability to prevent the passage of current. Dielectric materials include substances with electrical resistivity p > 10 7 Ohm * m. Due to their high electrical resistivity, they are used as electrical insulating materials.
If the band gap is 0.1 ... 0.3 eV, then electrons easily pass from the valence band to the conduction band due to external energy. Substances with controlled conductivity are semiconductors. The specific electrical resistance of semiconductors is p 10 9 ohm * m.
Semiconductor materials have conductivity, which can be used to control voltage, temperature, illumination, etc.
Depending on the structure and external conditions, materials can move from one class to another. For example, hard and liquid metals- conductors, and metal pairs - dielectrics; semiconductors germanium and silicon, which are typical under normal conditions, become conductors when exposed to high hydrostatic pressures; carbon in the diamond modification is a dielectric, and in the graphite modification it is a conductor.
The main property of matter in relation to the electric field is electrical conductivity, characterizing the ability of a material to conduct an electric current under the influence of a constant electric field, i.e. field, the voltage of which does not change with time.
Electrical conductivity is characterized by specific electrical conductivity and specific electrical resistance:
The values of electrical conductivity and electrical resistivity y different materials differ significantly. In the superconducting state, the specific electrical resistance of materials is zero, while for rarefied gases it tends to infinity.
It is easy to understand that the conductive properties of a crystal depend on the band gap separating the valence and conduction bands. The wider the band gap, the smaller (at a given temperature) the number of electrons that penetrate the conduction band, the lower the conductivity of the crystal.
Crystals can be good conductorseven if the valence band is filledentirely, if it is directly adjacent to the zoneconductivity or intersects with it (as a resultblurring of zones during the formation of a grating). With magnifiedby increasing the band gap, the crystals acquiredrestore the properties of insulators. Average valuesband gaps correspond tosemipro-water crystals.
Since the valence and conduction bands of crystalline conductors are not separated, electrons freely move from one allowed sublevel to another, acquiring an ordered speed under the action of an applied voltage. At the same time, with an increase in temperature, the resistance of the conductor increases due to a decrease in the free path of electrons in the crystal.
In semiconductor crystals, the conductivity is determined primarily by the number of electrons that have overcome the band gap and penetrated into the conduction band.
semiconductor materials
The chemical bond between the atoms of a semiconductor is carried out due to the interaction of valence electrons, while each atom receives from its neighbor in joint possession and gives it its valence electrons, which are missing before the formation of a stable eight-electron band. In this way, in an ideal semiconductor, all valence electrons rons participate in education chemical bond and free there are no electrons. This type of chemical bond is called a cova. tape.
On fig. 2.2 shows a diagram of the formation of a chemical bond in the crystal lattice of silicon, an atom of which has four valence electrons in a free isolated state, and an external electronic level each atom is filled with up to eight electrons, due to the joint ownership of the electrons of the four nearest neighboring atoms.
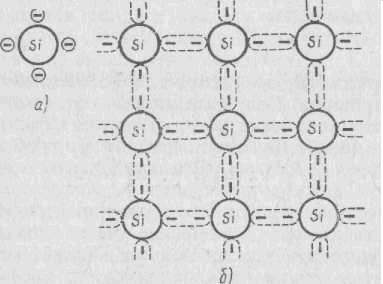
Figure 2.2 - Scheme of the formation of a chemical bond in the crystal lattice of silicon. Due to the small width (1 eV) of the band gap of a semiconductor, thermal vibrations of atoms are able to impart energy to valence electrons sufficient for the transition from the filled valence band to the free conduction band. Each such transition leads to the appearance of a pair of charge carriers: free electron in the conduction band and free energy state - holes- in the valence band. Under the action of the voltage applied to the crystal, the conduction electron moves "towards" electric field, and an electron in the valence band occupies a free level, freeing its level for another electron. This can be considered as the movement of a positive charge (hole) in the direction of the electric field.
Generation of pairs of free, i.e. capable of transferringmove under the influence of an applied voltage,charges makes the crystal capable of conducting electricityric current, and the electrical conductivity of such a crystalcalledown.
A semiconductor in which electrical conductivity is carried out due to the excitation of chemical bond electrons is calledown semiconductor.
Current carriers in such semiconductors are their own electrons and holes.
On fig. 2.3 shows a diagram of the crystal lattice of its own semiconductor. Upon excitation of its own valence electron, n-type of current carrier - free electron and R- type of current carrier - hole - unoccupied chemical bond.
Simultaneously with the formation of pairs of carriers, a part of the electrons from the conduction band spontaneously passes back to the valence band, emitting energy quanta. This process is called recombination steam. At a constant temperature, a dynamic equilibrium is established, which determines the concentration free electrons and holes (at a given temperature).
The higher the temperature, the higher the concentration of free charge carriers, the greater the intrinsic electrical conductivity of the crystal.
At a temperature of 0K, no carrier pairs are formed, and the crystal completely loses its own electrical conductivity. In this case, the electrons inside the crystal move randomly at high speeds (of the order of 10 6 m / s), but do not react to the applied external voltage.
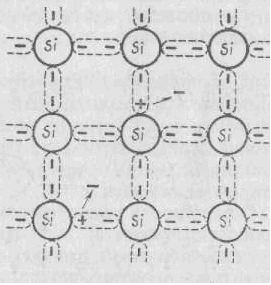
Figure 2.3 - Scheme of the crystal lattice of its own semiconductor.
Real crystals contain numerous defects in the crystal lattice: point, linear, bulk and surface. In places of violation of the periodic structure of the crystal (implantation of atoms into interstices), the binding energy of electrons with nuclei changes, as a result of which new energy levels arise, which can go beyond the valence band and be located in the band gap near the conduction band. This facilitates the transition of electrons to the conduction band.
Training and metodology complex... - to give basicsmaterials science, selection principles necessary materials, instill the skills of practical definition physical-mechanical... group chemical connections. 6.3.1. Classification of polymers and properties of polymers By origin polymers share ...
Evgeny Petrovich Prokopiev (rus)(eng) general list of publications antimatter and positronics positronics and nanotechnology positronics positron annihilation physics of complex systems synergetics materials science nanotechnology other related problems
DocumentProkopiev E.P. On surface states at the boundary section semiconductor-metal. M., 1985. 5 p. - Dep. in... processes. Third Russian Conference on materials science and physical-chemicalbasics technologies for obtaining doped silicon crystals...
The properties of an alloy depend on its structure. The main way to change the structure, and, consequently, the properties is heat treatment.
The basics of heat treatment were developed by D.K. Chernov. Later they were developed in the works of A.A. Bochvar, G.V. Kurdyumov, A.P. Gulyaev.
heat treatment called the thermal effect on the metal in order to give the metal the necessary mechanical and physical properties as a result of a change in the internal structure (structure) of the metal.
Most of the blanks (semi-finished products) and products made of steel and non-ferrous alloys are subjected to heat treatment. It is heat treatment that allows you to change the structure of the metal in the right direction and allows you to get required level hardness, strength, plasticity and other properties.
Heat treatment mode characterize the following main parameters: speed and mode of heating, Maximum temperature heating, holding time in the oven at the heating temperature, and cooling rate and mode.
Heat treatment is one of the most common modern technology ways to obtain the specified properties of the metal. Heat treatment is used either as an intermediate operation to improve the machinability of a semi-finished product by pressure, cutting, etc., or as a final operation. technological process, providing a given level of physical and mechanical properties of the part.
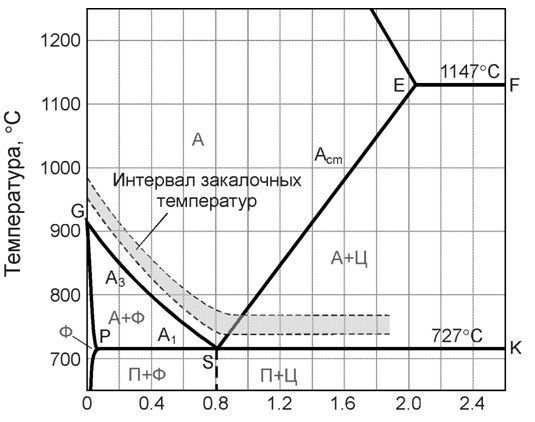
Heat treatment includes heating, holding and cooling of the metal, performed in a certain sequence under certain modes, in order to change the internal structure of the alloy and obtain the desired properties. Usually it can be schematically represented as a graph in the temperature-time axes (Fig. 21).
Heat treatment is subdivided into the actual thermal, chemical-thermal and thermomechanical (or deformation-thermal).
The actual heat treatment consists only in the thermal effect on the metal or alloy, chemical-thermal treatment - in a combination of thermal and chemical effects, thermomechanical - in a combination of thermal effect and plastic deformation.
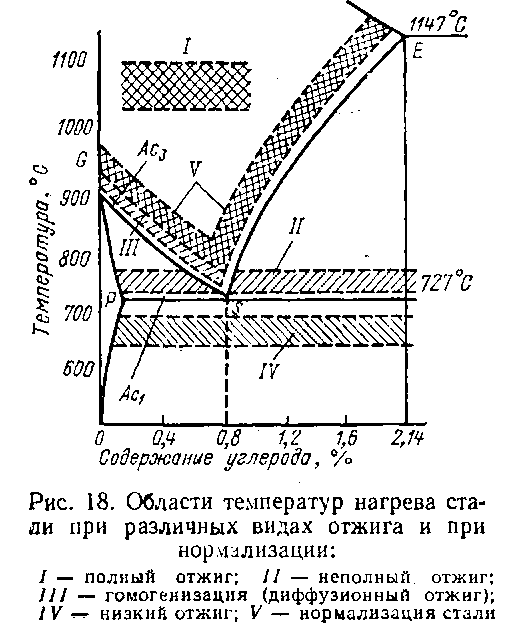
Fig.21. Heat Treatment Schedule
Actually heat treatment includes the following main types:
Annealing of the 1st kind
Annealing of the 2nd kind
Hardening with polymorphic transformation
Hardening without polymorphic transformation
Aging
These types of heat treatment apply to both steels and non-ferrous metals.
Annealing
Annealing- heat treatment, which consists in heating the metal to a certain temperature, holding and cooling with the furnace turned off (i.e. with a minimum possible speed, about 50-100 deg/hour).
Annealing of the 1st kind - is used for any metals and alloys. Its implementation is not due to phase transformations in the solid state. Heating during annealing of the first kind, increasing the mobility of atoms, partially or completely eliminates chemical inhomogeneity, reduces internal stresses. The heating temperature and holding time are of primary importance. Characterized by slow cooling
Varieties of annealing of the first kind are:
homogenization (diffusion)
· recrystallization;
· annealing to relieve internal stress after forging, welding, casting.
Homogenization (diffusion) annealing is a heat treatment in which the main process is the elimination of the consequences of dendritic segregation (chemical heterogeneity) in castings and ingots. It is a long exposure at high temperatures, at which diffusion processes take place, which did not have time to complete during crystallization. The approximate temperature for steels is -1100-1300 ° C for 20-50 hours, for aluminum alloys 420-450 ° C.
Recrystallization annealing is a heat treatment of a deformed metal, in which the main process is the recrystallization of the metal. This type of annealing eliminates deviations in the structure from the equilibrium state that occur during plastic deformation. During pressure treatment, especially cold, the metal is riveted, its strength increases, and plasticity decreases due to an increase in the density of dislocations in crystallites. When cold-worked metal is heated above a certain temperature, primary and then collective recrystallization develops, at which the dislocation density sharply decreases. As a result, the metal softens and becomes more ductile. Such annealing is used to improve workability by pressure and to give the metal the necessary combination of hardness, strength and ductility. As a rule, during recrystallization annealing, one strives to obtain a textureless material in which there is no anisotropy of properties. In the production of transformer steel sheets, recrystallization annealing is used to obtain the desired metal texture resulting from recrystallization.
Stress relief annealing is a heat treatment in which the main process is the complete or partial relaxation of residual stresses during heating and cooling. Stress-reducing annealing is applied to products in which, during pressure treatment, casting, welding, heat treatment, and other technological processes, unacceptably large residual stresses have arisen that are mutually balanced inside the body without the participation of external loads. Residual stresses can cause distortion of the shape and dimensions of the product during its processing, operation or storage in a warehouse. When the product is heated, the yield strength decreases and, when it becomes less than the residual stresses, they are quickly discharged by plastic flow in different layers of the metal.
Annealing of the second kind is the annealing of metals and alloys undergoing phase transformations in the solid state. This type of annealing is carried out for alloys in which there are polymorphic or eutectoid transformations, as well as variable solubility of the components in the solid state.
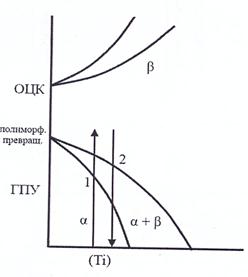
Fig. 22. Structure change during annealing of the second kind
Annealing of the second kind is carried out in order to obtain an equilibrium structure and prepare it for further processing. As a result of annealing, grain is crushed, ductility and toughness increase, strength and hardness decrease, and machinability improves. It is characterized by heating to temperatures above critical and very slow cooling, as a rule, together with a furnace or in air. In the latter case, the process is called normalization. Annealing of the 2nd kind is used most often for general refinement of the structure, softening and improvement of machinability.
Heat treatment of metals and alloys
Course work
on the topic: "Heat treatment of metals and alloys"
Introduction
Heat treatment is used at various stages of the production of machine parts and metal products. In some cases, it can be an intermediate operation that serves to improve the machinability of alloys by pressure, cutting, in others it is the final operation that provides the necessary set of indicators of mechanical, physical and operational properties of products or semi-finished products. Semi-finished products are subjected to heat treatment to improve the structure, reduce hardness (improve machinability), and parts - to give them certain required properties (hardness, wear resistance, strength, and others).
As a result of heat treatment, the properties of alloys can be changed over a wide range. The possibility of a significant increase in mechanical properties after heat treatment in comparison with the initial state makes it possible to increase the allowable stresses, reduce the size and weight of machines and mechanisms, and increase the reliability and service life of products. Improvement of properties as a result of heat treatment allows the use of alloys of simpler compositions, and therefore cheaper ones. Alloys also acquire some new properties, in connection with which the scope of their application is expanding.
Purpose and types of heat treatment
Thermal (heat) treatment is a process, the essence of which is the heating and cooling of products in certain modes, resulting in changes in the structure, phase composition, mechanical and physical properties of the material, without changing the chemical composition.
The purpose of heat treatment of metals is to obtain the required hardness, improve the strength characteristics of metals and alloys. Heat treatment is divided into thermal, thermomechanical and chemical-thermal. Heat treatment - only thermal action, thermomechanical - a combination of thermal action and plastic deformation, chemical-thermal - a combination of thermal and chemical effects. Heat treatment, depending on the structural state obtained as a result of its application, is divided into annealing (first and second kind), hardening and tempering.
Annealing
Annealing is a heat treatment that consists in heating the metal to certain temperatures, holding and then very slow cooling together with the furnace. They are used to improve the processing of metals by cutting, to reduce hardness, to obtain a granular structure, as well as to relieve stress, partially (or completely) eliminates all kinds of inhomogeneities that were introduced into the metal during previous operations (machining, pressure treatment, casting, welding), improves the steel structure.
Annealing of the first kind. This is annealing during which phase transformations do not occur, and if they occur, they do not affect the final results provided for by its intended purpose. There are the following types of annealing of the first kind: homogenization and recrystallization.
Homogenization is annealing with a long exposure at a temperature above 950ºC (usually 1100–1200ºC) in order to equalize the chemical composition.
Recrystallization is the annealing of work-hardened steel at a temperature exceeding the temperature of the beginning of recrystallization, in order to eliminate hardening and obtain a certain grain size.
Annealing of the second kind. This is annealing, in which phase transformations determine its intended purpose. The following types are distinguished: complete, incomplete, diffusion, isothermal, light, normalized (normalization), spheroidizing (for granular perlite).
Full annealing is carried out by heating the steel 30–50 °C above the critical point, holding at this temperature and slowly cooling to 400–500 °C at a rate of 200 °C per hour for carbon steels, 100 °C per hour for low-alloy steels and 50 °C. C per hour for high alloy steels. The steel structure after annealing is balanced and stable.
Incomplete annealing is carried out by heating steel to one of the temperatures in the transformation range, holding and slow cooling. Incomplete annealing is used to reduce internal stresses, lower hardness and improve machinability.
Diffusion annealing. The metal is heated to temperatures of 1100–1200ºC, since in this case the diffusion processes necessary to equalize the chemical composition proceed more fully.
Isothermal annealing is as follows: the steel is heated and then rapidly cooled (often by transfer to another furnace) to a temperature that is 50–100 °C below the critical one. Mainly used for alloy steels. Economical, since the duration of conventional annealing (13 - 15) h, and isothermal annealing (4 - 6) h
Spheroidizing annealing (for granular pearlite) consists in heating the steel above the critical temperature by 20–30 °C, holding at this temperature and slow cooling.
Bright annealing is carried out according to the modes of complete or incomplete annealing using protective atmospheres or in furnaces with partial vacuum. It is used to protect the metal surface from oxidation and decarburization.
Normalization - consists in heating the metal to a temperature of (30–50) ºС above the critical point and subsequent cooling in air. The purpose of normalization is different depending on the composition of the steel. Instead of annealing, low carbon steels are normalized. For medium carbon steels, normalization is used instead of quenching and high tempering. High-carbon steels are subjected to normalization in order to eliminate the cementite network. Normalization followed by high tempering is used instead of annealing to correct the structure of alloyed steels. Normalization is a more economical operation than annealing, as it does not require cooling along with the furnace.
hardening
Hardening is heating to the optimum temperature, holding and subsequent rapid cooling in order to obtain a non-equilibrium structure.
As a result of hardening, the strength and hardness increase and the ductility of the steel decreases. The main parameters during hardening are heating temperature and cooling rate. The critical quenching rate is the cooling rate that provides the formation of a structure - martensite or martensite and residual austenite.
Depending on the shape of the part, the steel grade and the required set of properties, various hardening methods are used.
Hardening in one cooler. The part is heated to the hardening temperature and cooled in one coolant (water, oil).
Hardening in two environments (interrupted hardening) is a hardening in which the part is cooled sequentially in two environments: the first medium is a coolant (water), the second is air or oil.
Step hardening. The part heated to the hardening temperature is cooled in molten salts, after holding for the time necessary to equalize the temperature over the entire section, the part is cooled in air, which helps to reduce hardening stresses.
Isothermal hardening, like step hardening, is carried out in two cooling media. The temperature of the hot medium (salt, nitrate or alkaline baths) is different: it depends on the chemical composition of the steel, but it is always 20–100 °C higher than the martensitic transformation point for a given steel. Final cooling to room temperature is carried out in air. Isothermal hardening is widely used for parts made of high-alloy steels. After isothermal hardening, the steel acquires high strength properties, that is, a combination of high toughness with strength.
Hardening with self-tempering is widely used in tool making. The process consists in the fact that the parts are kept in a cooling medium not until completely cooled, but at a certain moment they are removed from it in order to save a certain amount of heat in the core of the part, due to which the subsequent tempering is carried out.
Vacation
Tempering of steel is the final operation of heat treatment, which forms the structure and, consequently, the properties of steel. Tempering consists in heating steel to different temperatures (depending on the type of tempering, but always below the critical point), holding at this temperature and cooling at different rates. The purpose of tempering is to relieve internal stresses that arise during the hardening process and obtain the necessary structure.
Depending on the heating temperature of the hardened part, there are three types of tempering: high, medium and low.
High tempering is carried out at heating temperatures above 350–600 °C, but below the critical point; such tempering is used for structural steels.
Average tempering is carried out at heating temperatures of 350 - 500 °C; such tempering is widely used for spring and spring steels.
Low tempering is carried out at temperatures of 150–250 °С. The hardness of the part after hardening almost does not change; Low tempering is used for carbon and alloy tool steels where high hardness and wear resistance are required.
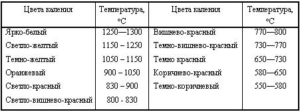
The tempering control is carried out by the tempering colors that appear on the surface of the part.
Aging
Aging is a process of changing the properties of alloys without a noticeable change in the microstructure. There are two types of aging: thermal and deformation.
Thermal aging occurs as a result of changes in the solubility of carbon in iron depending on temperature.
If the change in hardness, ductility and strength occurs at room temperature, then such aging is called natural.
If the process proceeds at an elevated temperature, then aging is called artificial.
Deformation (mechanical) aging proceeds after cold plastic deformation.
Cold treatment
A new kind of heat treatment to increase the hardness of steel by converting the retained austenite of hardened steel into martensite. This is done by cooling the steel to the temperature of the lower martensitic point.
Surface hardening methods
Surface hardening is a heat treatment process, which is heating the surface layer of steel to a temperature above the critical temperature and subsequent cooling in order to obtain a martensite structure in the surface layer.
There are the following types: induction hardening; quenching in an electrolyte, quenching by heating with high frequency currents (HFC), quenching with flame heating.
Induction hardening is based on a physical phenomenon, the essence of which is that a high-frequency electric current, passing through a conductor, creates an electromagnetic field around it. Eddy currents are induced on the surface of a part placed in this field, causing the metal to heat up to high temperatures. This makes it possible for phase transformations to occur.
Depending on the heating method, induction hardening is divided into three types:
simultaneous heating and hardening of the entire surface (used for small parts);
sequential heating and hardening of individual sections (used for crankshafts and similar parts);
continuous-sequential heating and hardening by movement (used for long parts).
Gas flame hardening. The process of gas-flame hardening consists in the rapid heating of the surface of the part with an oxy-acetylene, oxy-fuel or oxygen-kerosene flame to the hardening temperature, followed by cooling with water or an emulsion.
Hardening in electrolyte. The process of hardening in an electrolyte is as follows: the part to be hardened is lowered into a bath with electrolyte (5–10% solution of calcined salt) and a current of 220–250 V is passed through. As a result, the part is heated to high temperatures. The part is cooled either in the same electrolyte (after turning off the current) or in a special hardening tank.
Thermomechanical processing
Thermomechanical treatment (T.M.O.) is a new method of strengthening metals and alloys while maintaining sufficient plasticity, combining plastic deformation and hardening heat treatment (quenching and tempering). There are three main methods of thermomechanical processing.
Low-temperature thermomechanical processing (L.T.M.O.) is based on stepwise hardening, that is, plastic deformation of steel is carried out at temperatures of relative stability of austenite, followed by hardening and tempering.
High-temperature thermomechanical processing (H.T.M.O.) in this case, plastic deformation is carried out at austenite stability temperatures, followed by quenching and tempering.
Preliminary thermomechanical processing (P.T.M.O) deformation in this case can be carried out at temperatures N.T.M.O and V.T.M.O or at a temperature of 20ºC. Further, the usual heat treatment is carried out: hardening and tempering.
Purpose and types of chemical-thermal treatment
Chemical-thermal treatment is a process that is a combination of thermal and chemical effects in order to change the composition, structure and properties of the surface layer of steel.
The purpose of chemical-thermal treatment is to increase surface hardness, wear resistance, endurance limit, corrosion resistance, heat resistance (scaling resistance), acid resistance.
The following types of chemical-thermal treatment have received the greatest application in industry: carburizing; nitrocarburizing; nitriding; cyanidation; diffusion metallization.
Cementation is a process of surface carbonization produced for the purpose of surface hardening of parts.
Depending on the carburizer used, carburizing is divided into three types: carburizing with a solid carburizer; gas carburizing (methane, propane, natural gas).
Gas carburizing. Parts are heated to 900–950ºC in special hermetically sealed furnaces, into which a cementing carbon-containing gas [natural (natural) or artificial) is supplied in a continuous stream.
The carburizing process in a solid carburetor is as follows. Parts packed in a box together with a carburetor (a mixture of charcoal with an activator) are heated to a certain temperature and kept at this temperature for a long time, then cooled and subjected to heat treatment.
Parts made of carbon and alloy steel with a carbon content of not more than 0.2% are carburized by any of the methods discussed above. Carburizing of alloy steels containing Cr, W, V carbide-forming elements gives particularly good results: in addition to increasing surface hardness and wear resistance, they also increase fatigue strength.
Nitriding- this is the process of saturation of the surface layer of various metals and alloys, steel products or parts with nitrogen when heated in an appropriate environment. Increases the hardness of the surface of the product, endurance, wear resistance, increase corrosion resistance.
Cyanidation– .saturation of the surface layer of products simultaneously with carbon and nitrogen.
Depending on the medium used, cyanidation is distinguished: in solid media; in liquid media; in gas environments.
Depending on the heating temperature, cyanidation is divided into low-temperature and high-temperature cyanidation.
Cyanidation in liquid media is carried out in molten salt baths.
Cyaniding in gaseous media (nitrocarburizing). The process of simultaneously saturating the surface of a part with carbon and nitrogen. To do this, the parts are heated in an environment consisting of carburizing gas and ammonia, that is, nitrocarburizing combines the processes of gas carburizing and nitriding.
Diffusion saturation with metals and metalloids
There are and are used in industry methods for saturating the surface of parts with various metals (aluminum, chromium, etc.) and metalloids (silicon, boron, etc.). The purpose of such saturation is to increase scale resistance, corrosion resistance, acid resistance, hardness and wear resistance of parts. As a result, the surface layer acquires special properties, which makes it possible to save alloying elements.
Aluminizing– the process of saturation of the surface layer of steel with aluminum to increase heat resistance (scale resistance) and resistance to atmospheric corrosion.
Aluminizing is carried out in powder mixtures, in baths with molten aluminum, in a gaseous medium and by spraying liquid aluminum.
Chrome plating- the process of saturation of the surface layer of steel with chromium to increase corrosion resistance and heat resistance, and in the case of chromium plating of high-carbon steels - to increase hardness and wear resistance.
Siliconization– the process of saturation of the surface layer of the part with silicon to increase corrosion resistance and acid resistance. Silicification is applied to parts made of low- and medium-carbon steels, as well as malleable and high-strength cast irons.
Boriding- the process of saturation of the surface layer of the part with boron. The purpose of boriding is to increase hardness, resistance to abrasive wear and corrosion in aggressive environments, heat resistance and heat resistance of steel parts. There are two methods of boriding: liquid electrolysis and gas boriding.
Sulfidation– the process of saturation of the surface layer of steel parts with sulfur to improve extreme pressure properties and increase wear resistance of parts.
Sulfocyanation– the process of surface saturation of steel parts with sulfur, carbon and nitrogen. The combined effect of sulfur and nitrogen in the surface layer of the metal provides higher extreme pressure properties and wear resistance compared to saturation with sulfur alone.
Heat treatment of cast iron
Heat treatment of cast iron is carried out in order to relieve internal stresses that arise during casting and cause changes in the size and shape of the casting over time, reduce hardness and improve machinability, and improve mechanical properties. Cast iron is subjected to annealing, normalization, hardening and tempering, as well as some types of chemical-thermal treatment (nitriding, aluminizing, chromium plating).
Stress Relief Annealing. Cast irons are subjected to this annealing at the following temperatures: gray cast iron with lamellar graphite 500 - 570ºС; ductile iron with nodular graphite 550 - 650ºС; low-alloy cast iron 570 - 600ºС; high-alloy cast iron 620 - 650ºС. During this annealing, phase transformations do not occur, but internal stresses are removed, viscosity increases, warping and cracking during operation are excluded.
Softening annealing (low-temperature graphitising annealing). Carried out to improve machinability and increase ductility. It is carried out by prolonged exposure at 680 - 700ºC or slow cooling of castings at 760 - 700ºC. For details of a complex configuration, cooling is slow, and for parts of a simple shape, it is accelerated.
Annealing graphitising, as a result of which malleable iron is obtained from white cast iron.
Normalization used to increase the bound carbon, increase the hardness, strength and wear resistance of gray, ductile and high-strength cast irons. During normalization, cast iron (castings) is heated above the temperatures of the transformation range of 850 - 950ºС and, after exposure, is cooled in air.
hardening subject gray, malleable and ductile iron to increase hardness, strength and wear resistance. According to the method of execution, hardening of cast iron can be volumetric continuous, isothermal and surface.
During volumetric continuous hardening, cast iron is heated to a temperature of 850 - 950ºC. Then stand for heating and complete dissolution of carbon. Cooling is carried out in water or oil. After quenching, tempering is carried out at a temperature of 200 - 600ºС. As a result, the hardness, strength and wear resistance of cast iron increase.
During isothermal quenching, cast irons are heated in the same way as during volumetric continuous quenching, held for 10 to 90 minutes and cooled in molten salt at 200-400ºC, and after holding, cooled in air.
Surface hardening with heating of the surface layer with an oxygen-acetylene flame, high-frequency currents or in an electrolyte. Heating temperature 900 - 1000ºС. Cooling in water, oil or oil emulsion.
Aging used to stabilize the dimensions of cast iron parts, prevent warping and relieve internal stresses. Typically, aging is carried out after rough machining. There are two types of aging: natural and artificial.
Natural aging is carried out outdoors or indoors. Products after casting are aged for 6 - 15 months.
Artificial aging is carried out at elevated temperatures; duration - several hours. In case of artificial aging, cast iron castings are loaded into a furnace heated to 100 - 200 ° C, heated to a temperature of 550 - 570 ° C at a rate of 30 - 60 ° C per hour, kept for 3 - 5 hours and cooled together with the furnace at a rate of 20 - 40 ° C per hour to a temperature of 150 - 200ºC, and then cooled in air.
Chemical-thermal treatment of cast iron
To increase surface hardness and wear resistance, gray cast irons are subjected to nitriding. More often gray pearlitic cast irons alloyed with chromium, molybdenum, and aluminum are nitrided. Nitriding temperature 550 - 580ºС, exposure time 30 - 70 hours. In addition to nitriding, increasing the surface hardness and wear resistance of alloyed gray pearlitic cast iron can be achieved by gas and liquid cyanidation at a temperature of 570ºC. To increase heat resistance, cast iron castings can be subjected to aluminizing, and to obtain high corrosion resistance in acids, siliconized.
Heat treatment of non-ferrous metal alloys
Aluminum alloys
Aluminum alloys are subjected to three types of heat treatment: annealing, hardening and aging. Main types annealing are: diffusion, recrystallization and thermally hardened alloys.
Homogenization is used to equalize the chemical microheterogeneity of solid solution grains. To perform homogenization, aluminum alloys are heated to 450 - 520ºС and kept at these temperatures from 4 to 40 hours; after exposure - cooling together with the furnace or into the air. As a result, the structure becomes more homogeneous and plasticity increases.
Recrystallization annealing for aluminum and alloys based on it is used much more widely than for steel. This is explained by the fact that metals such as aluminum and copper, as well as many alloys based on them, are not hardened by hardening and an increase in mechanical properties can only be achieved by cold working, and an intermediate operation in such processing is recrystallization annealing. Temperature of recrystallization annealing of aluminum alloys 300 - 500ºC exposure 0.5 - 2 hours.
Annealing of thermally hardened alloys is used to completely remove the hardening; it is carried out at temperatures of 350 - 450 ° C with an exposure of 1 - 2 hours and subsequent rather slow cooling.
After hardening the strength of the alloy increases slightly, but the ductility does not change. After quenching, aluminum alloys are subjected to aging, at which the decomposition of a supersaturated solid solution occurs.
Wrought aluminum alloys
In the hardened state, duralumins are ductile and easily deformed. After hardening and natural or artificial aging, the strength of duralumin increases dramatically.
Cast aluminum alloys
For cast aluminum alloys, various types of heat treatment are used depending on the chemical composition. For hardening, cast aluminum alloys are subjected to quenching to obtain a supersaturated solid solution and artificial aging, as well as only quenching without aging to obtain a stable solid solution in the quenched state.
magnesium alloys
Magnesium alloys, like aluminum, are subjected to annealing, hardening and aging. To equalize the chemical microheterogeneity of the grains of the solid solution by diffusion, magnesium alloy ingots are subjected to homogenization at temperatures of 350–400°C with an exposure of 18–24 hours. Semi-finished products of wrought magnesium alloys are subjected to recrystallization annealing at a temperature of ≈ 350ºC, and also at lower temperatures of 150 - 250ºC annealing to relieve residual stresses.
Magnesium alloys are hardened, or hardened and artificially aged. At a temperature of 20 ° C, no changes occur in hardened magnesium alloys, that is, they are not subject to natural aging.
Copper and copper alloys
Heat treatment of copper. The deformation of copper is accompanied by an increase in its strength and a decrease in ductility. To increase plasticity, copper is subjected to recrystallization annealing at 500 - 600ºC, as a result of which plasticity increases sharply, and strength decreases.
Heat treatment of brass. They are subjected only to recrystallization annealing at 600 - 700ºC (to remove hardening). Brass is cooled during annealing in air or to accelerate cooling and better separation of scale in water. For brass parts that have residual stresses after deformation, in a humid atmosphere, the phenomenon of spontaneous cracking is characteristic. To avoid this, brass parts are subjected to low-temperature annealing at 200 - 300 C, as a result of which residual stresses are removed, and hardening remains. Low-temperature annealing is especially necessary for aluminum brasses, which are prone to spontaneous cracking.
Heat treatment of bronzes. To equalize the chemical composition of the bronze is subjected to homogenization at 700 - 750ºC, followed by rapid cooling. To relieve internal stresses, castings are annealed at 550°C. To restore plasticity between operations of cold working by pressure, they are subjected to recrystallization annealing at 600 - 700ºС.
Aluminum bronzes with an aluminum content of 8 to 11%, which experience phase recrystallization during heating and cooling, can be hardened. As a result of hardening, strength and hardness increase, but ductility decreases. Hardening is followed by tempering at 400 - 650º C, depending on the required properties. Also subjected to homogenization, and deformable semi-finished products - recrystallization annealing at 650 - 800ºC.
Beryllium bronze is hardened in water from a temperature of 760 - 780ºС; in this case, the excess phase does not have time to precipitate, and after quenching, the alloy consists of a supersaturated solid solution and has low hardness and strength and high ductility. After quenching, tempering (aging) is carried out at 300 - 350ºС for 2 hours. To increase the stability of the supersaturated solid solution and facilitate hardening, beryllium bronzes are additionally alloyed with nickel.
titanium alloys
Titanium alloys are subjected to recrystallization annealing and annealing with phase recrystallization, as well as hardening by heat treatment - quenching and aging. To improve wear resistance and scuff resistance, titanium alloys are subjected to nitriding, carburizing, or oxidation.
Recrystallization annealing is used for titanium and alloys to remove work hardening after cold working. The temperature of recrystallization annealing is 520 - 850ºC, depending on the chemical composition of the alloy and the type of semi-finished product.
Annealing with phase recrystallization is used to reduce hardness, increase ductility, grain refinement, and eliminate structural heterogeneity. Apply simple, isothermal and double annealing; heating temperature during annealing 750 - 950ºС depending on the alloy.
During isothermal annealing, after holding at the annealing temperature, the parts are cooled to 500 - 650ºС (depending on the alloy) in the same furnace or transferred to another furnace and kept for a certain time, and cooled in air. With isothermal annealing, the annealing time is shortened, and the ductility is higher.
In double annealing, the parts are heated to the annealing temperature, held and cooled in air. Then I heat it up to 500 - 650ºС again, hold it and cool it in air. Double annealing, as compared to isothermal annealing, increases the tensile strength with a slight decrease in ductility and reduces the processing time.
Of all types of chemical-thermal treatment of titanium alloys, nitriding is most widely used, carried out in a nitrogen environment or in a mixture of nitrogen and argon at temperatures of 850–950 C for 10–50 hours. Parts made of titanium alloys after nitriding have good anti-friction properties.
Conclusion
Heat treatment is one of the main, most important operations of the general technological cycle of processing, on the correct implementation of which the quality (mechanical and physico-chemical properties) of manufactured parts of machines and mechanisms, tools and other products depends. Technological processes of heat treatment of gray and white cast irons, non-ferrous metal alloys have been developed and rationalized.
A promising direction for improving heat treatment technology is the installation of heat treatment units in machine shops, the creation of automatic lines with the inclusion of heat treatment processes in them, as well as the development of methods that improve the strength properties of parts, their reliability and durability.
Literature
B.V. Zakharov. V.N. Berseneva "Progressive technological processes and equipment for heat treatment of metals" M. " graduate School» 1988
V.M. Zuev "Heat treatment of metals" M. Higher school 1986
B.A. Kuzmin "Technology of metals and structural materials" M. "Engineering" 1981
V.M. Nikiforov "Technology of metals and structural materials" M. "Higher School" 1968
A.I. Samohotsky N.G. Parfenovskaya "Technology of heat treatment of metals" M. Engineering 1976
The choice of material and the development of the technological process of heat treatment of the die
Working condition of die, threading tool for cutting external thread manually or on a machine tool. Characteristics of steel, its chemical, mechanical and other properties. Methods for monitoring heat treatment modes and product quality.
Steel grade 18KhGT: chromium-manganese steel contains 0.18% carbon, up to 1% chromium, manganese. The sequence of operations of preliminary and final heat treatment of parts. Mode of operations of preliminary and final heat treatment of parts.
Theoretical basis heat treatment of steel. Diffusion and recrystallization annealing. Quenching is a heat treatment in which the steel acquires a non-equilibrium structure and the hardness of the steel increases. Application of heat treatment in practice.
The essence of the purpose of the cutter and its application. Analysis of technological properties and chemical composition of high-speed steels. Stages of the technological process of preliminary and hardening heat treatment, selection of fixtures, defects and their elimination.
Fundamentals of technology of heat treatment of metals and alloys. Heat treatment is a stage in the technological process of manufacturing parts. Improving the machinability of materials by pressure or cutting. Formation of technical and electrical properties.
Description of the procedure for applying the hardening of carbon steels and determining the hardening temperature according to the task. Calculation of the required hardening time. Appointment of annealing and determination of its time according to the task. Protocol rules.
Formation of material properties of the part. Impact machining on the material properties of the workpieces. Influence of cutting fluid (coolant).
Deciphering the steel grade. The nature of the influence of carbon and alloying elements of a given steel on the position of critical points. Selection and justification of the sequence of operations of preliminary and final heat treatment of parts. The mode of heat treatment of parts.
Theory of heat treatment. Transformations in steel during heating and cooling. Annealing and normalization. Heat treatment defects. Defects during annealing and normalization. Hardening defects. Chemical-thermal treatment and surface hardening of steel.
Theory and technology of heat treatment. Types of heat treatment. Annealing, normalization, hardening, aging, improvement. Chemical-thermal treatment. Her types. Composite materials.
Rules for machining parts by cutting - removal from the workpiece using cutting tool allowance, consistently bringing its shape and dimensions to the required ones, turning it into ready product. Surface quality control by chemical-thermal treatment.
Familiarization with the methodology for developing the technological process of heat treatment of parts: cars, tractors and agricultural machines. Deciphering the grade of a given steel, a description of its microstructure, mechanical properties before heat treatment.
Steel grade 20HNR - chromium-nickel steel with a carbon content of 0.20%, up to 1% chromium, nickel and boron. The mode of operations of preliminary and final heat treatment of parts is the heating temperature and the microstructure in the heated state, the cooling medium.