A message on the topic of halogen compounds. Halogens and their compounds. Biological significance of halogens and their compounds
Read also
Halogens (from Greek. halos - salt and genes - generator) - elements of the main subgroup VII groups periodic table: fluorine, chlorine, bromine, iodine, astatine.
In the free state, halogens form substances consisting of diatomic molecules F 2, Cl 2, Br 2, I 2.
BEING IN NATURE
Halogens occur in nature only in the form of compounds.
Fluorine occurs exclusively in the form of salts dispersed in various rocks. The total fluorine content in the earth's crust is 0.02% of atoms. Fluorine minerals are of practical importance: CaF 2 - fluorspar, Na 2 AlF 6 - cryolite, Ca 5 F(PO 4) 3 - fluorapatite.
The most important natural compound chlorine
is sodium chloride (halite), which serves as the main raw material for the production of other chlorine compounds. The main mass of sodium chloride is found in the water of the seas and oceans. The waters of many lakes also contain significant amounts of NaCl - such as lakes Elton and Baskunchak. There are other chlorine compounds, for example, KCl - sylvinite, MgCl 2 *KCl*6HO - carnallite, KCl*NaCl - sylvinite.
Bromine found in nature in the form of sodium and potassium salts along with chlorine salts, as well as in the water of salt lakes and seas. Metal bromides are found in sea water. In underground drilling waters of industrial importance, the bromine content ranges from 170 to 700 mg/l. Total bromine content in earth's crust 3*10-5% atoms.
Connections iodine
are present in sea water, but in such small quantities that their direct isolation from water is very difficult. However, there are some algae that accumulate iodine in their tissues, such as kelp. The ash of these algae serves as a raw material for the production of iodine. A significant amount of iodine (from 10 to 50 mg/l.) is contained in underground drilling waters. The iodine content in the earth's crust is 4*10-6% atoms. There are minor deposits of iodine salts - KIO 3 and KIO 4 - in Chile and Bolivia.
total weight astata on globe estimated to not exceed 30 g.
Table. Electronic structure and some properties of halogen atoms and molecules
|
Symbol element |
|||||
|
Ordinal Number |
|||||
|
Structure external electronic layer |
2s 2 2p 5 |
3s 2 3p 5 |
4s 2 4p 5 |
5 s 2 5 p 5 |
6 s 2 6 p 5 |
|
Relative electro negativity (EO) |
4,0 |
3,0 |
2,8 |
2,5 |
~2,2 |
|
Atomic radius, nm |
0,064 |
0,099 |
0,114 |
0,133 |
– |
|
Degrees oxidation |
1, +1, +3, |
– |
|||
|
State of aggregation |
Pale green |
Green-yellow. |
Buraya |
Dark violet |
Black |
|
t °pl.(°С) |
219 |
101 |
114 |
227 |
|
|
t °boiling (°С) |
183 |
185 |
317 |
||
|
ρ (g/cm3) |
1,51 |
1,57 |
3,14 |
4,93 |
– |
|
Solubility in water (g/100g water) |
reacts |
2,5: 1 |
3,5 |
0,02 |
– |
|
Name |
Atomic structure diagram |
Electronic formula |
|
Fluorine |
F +9) 2) 7 |
… 2s 2 2p 5 |
|
Chlorine |
Cl +17) 2) 8) 7 |
… 3s 2 3p 5 |
|
Bromine |
Br +35) 2) 8) 18) 7 |
…4s 2 4p 5 |
|
Iodine |
I +53) 2) 8) 18) 18) 7 |
…5s 2 5p 5 |
1) The general electronic configuration of the outer energy level is nS 2 nP 5 .
2) With an increase in the atomic number of elements, the radii of atoms increase, electronegativity decreases, and metallic properties(metallic properties increase); halogens are strong oxidizing agents, the oxidizing ability of elements decreases with increasing atomic mass.
3) As the atomic mass increases, the color becomes darker, the melting and boiling points and density increase.
OBTAINING HALOGENS
1. Electrolysis of solutions and melts of halides:
2NaCl + 2H 2 O = Cl 2 + H 2 + 2NaOH
2 KF = 2 K + F 2 (the only way to get F 2)
2. Oxidation of hydrogen halides:
2 KMnO 4 +16 HCl =2 KCl +2 MnCl 2 +5 Cl 2 +8 H 2 O – Laboratory method for producing chlorine
14HBr+K 2 Cr 2 O 7 =2KBr+2CrBr 3 +3Br 2 +7H 2 O
MnO 2 + 4 HHal = MnHal 2 + Hal 2 + 2 H 2 O – Laboratory - (For the production of chlorine, bromine, iodine)
3. Industrial method - oxidation with chlorine (for bromine and iodine):
2KBr+Cl 2 =2KCl+Br 2
2KI + Cl 2 = 2KCl + I 2
Chemical properties
Let's look at the properties of halogens using chlorine as an example:
|
1. Interaction with metals |
2K + Cl 2 →2KCl experiment Mg + Cl 2 → MgCl 2 |
|
2.Reactions with non-metals |
H 2 + Cl 2 → 2HCl |
|
3. Interaction with alkalis in the cold |
2NaOH + Cl 2 → NaCl + NaClO + H 2 O |
|
4. Interaction with alkalis when heated |
6NaOH + 3Cl 2 → 5NaCl + NaClO 3 + 3H 2 O |
|
5. Displacement of less active halogens from halides |
2KBr + Cl 2 → 2KCl + Br 2 |
|
6. With water |
H 2 O + Cl 2 ↔ HCl + HClO (chlorine water) |
APPLICATION OF HALOGENS
|
Fluorine |
is widely used as a fluorinating agent in the production of various fluorides (SF 6, BF 3, WF 6 and others), including compounds of the noble gases xenon (Xe) and krypton (Kr). Uranium hexafluoride UF 6 is used to separate uranium (U) isotopes. Fluorine is used in the production of Teflon, other fluoroplastics, fluorine rubber, fluorine-containing organic matter and materials that are widely used in technology, especially in cases where resistance to aggressive environments is required, high temperature and so on. |
|
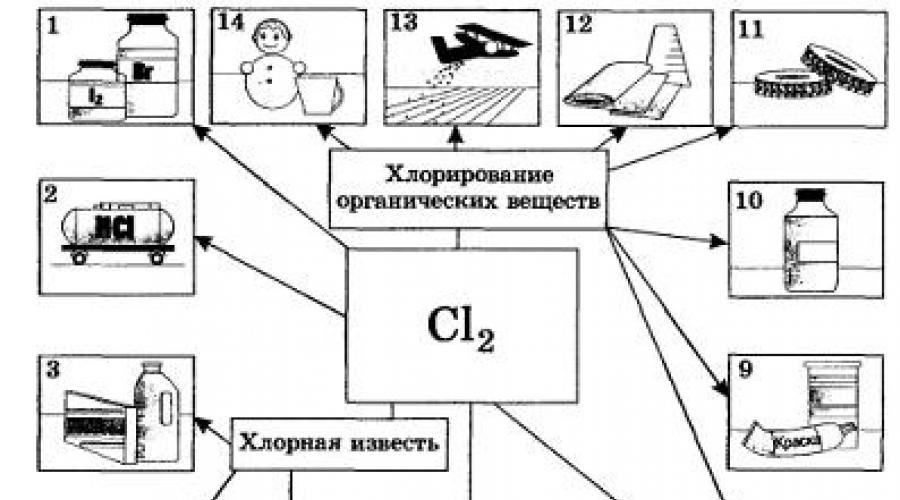
Chlorine |
used in the production of chlorine-containing organic compounds (60-75%), inorganic substances(10-20%), for bleaching cellulose and fabrics (5-15%), for sanitary needs and disinfection (chlorination) of water. |
|
Bromine |
bromine is used in the preparation of a number of inorganic and organic substances in analytical chemistry. Bromine compounds are used as fuel additives, pesticides, flame retardants, and in photography. Widely known to contain bromine medications. It should be noted that the common expression: “the doctor prescribed bromine a tablespoon after meals” means, of course, only that an aqueous solution of sodium (or potassium) bromide was prescribed, and not pure bromine. The calming effect of bromide drugs is based on their ability to enhance inhibition processes in the central nervous system. |
|
Iodine |
iodine is used to obtain high-purity titanium (Ti), zirconium (Zr), hafnium (Hf), niobium (Nb) and other metals (so-called iodide refining of metals). During iodide refining, the original metal with impurities is converted into the form of volatile iodides, and then the resulting iodides are decomposed on a hot thin thread. The thread is made of pre-cleaned metal, which is subjected to refining. Its temperature is selected such that only the iodide of the metal being purified can decompose on the filament, while the remaining iodides remain in the vapor phase. |
DEFINITION
Halogens– elements of group VII A – fluorine (F), chlorine (Cl), bromine (Br) and iodine (I).
Electronic configuration of the outer energy level of halogens ns 2 np 5. Since halogens lack only one electron before completing the energy level, in ORR they most often exhibit the properties of oxidizing agents. Oxidation states of halogens: from “-1” to “+7”. The only element of the halogen group, fluorine, exhibits only one oxidation state “-1” and is the most electronegative element. Halogen molecules are diatomic: F 2, Cl 2, Br 2, I 2.
Chemical properties of halogens
With increasing charge of the nucleus of an atom of a chemical element, i.e. when moving from fluorine to iodine, the oxidizing ability of halogens decreases, which is confirmed by the ability to displace lower halogens by higher ones from hydrohalic acids and their salts:
Br 2 + 2HI = I 2 + 2HBr;
Cl 2 + 2KBr = Br 2 + 2KCl.
Fluorine has the greatest chemical activity. Most chemical elements even with room temperature interacts with fluorine, releasing a large number of warmth. Even water burns in fluorine:
2H 2 O + 2F 2 = 4HF + O 2.
Free chlorine is less reactive than fluorine. It does not react directly with oxygen, nitrogen and noble gases. It interacts with all other substances like fluorine:
2Fe + Cl 2 = 2FeCl 3;
2P + 5Cl 2 = 2PCl 5.
When chlorine interacts with water in the cold, a reversible reaction occurs:
Cl 2 + H 2 O↔HCl +HClO.
The mixture of reaction products is called chlorine water.
When chlorine interacts with alkalis in the cold, mixtures of chlorides and hypochlorites are formed:
Cl 2 + Ca(OH) 2 = Ca(Cl)OCl + H 2 O.
When chlorine is dissolved in a hot alkali solution, the following reaction occurs:
3Cl 2 + 6KOH = 5KCl + KClO 3 + 3H 2 O.
Bromine, like chlorine, dissolves in water and, partially reacting with it, forms the so-called “bromine water,” while iodine is practically insoluble in water.
Iodine differs significantly in chemical activity from other halogens. It does not react with most non-metals, and reacts slowly with metals only when heated. The interaction of iodine with hydrogen occurs only with strong heating; the reaction is endothermic and highly reversible:
H 2 + I 2 = 2HI - 53 kJ.
Physical properties of halogens
At no. fluorine is a light yellow gas with a pungent odor. Poisonous. Chlorine is a light green gas, just like fluorine, it has a pungent odor. Highly poisonous. At high blood pressure and room temperature easily transforms into liquid state. Bromine is a heavy liquid of red-brown color with a characteristic unpleasant pungent odor. Liquid bromine, as well as its vapors, are highly toxic. Bromine is poorly soluble in water and well in non-polar solvents. Iodine is a dark gray solid with a metallic sheen. Iodine vapor is purple. Iodine easily sublimes, i.e. transforms into a gaseous state from a solid, while bypassing the liquid state.
Production of halogens
Halogens can be obtained by electrolysis of solutions or melts of halides:
MgCl 2 = Mg + Cl 2 (melt).
Most often, halogens are obtained by the oxidation reaction of hydrohalic acids:
MnO 2 + 4HCl = MnCl 2 + Cl 2 + 2H 2 O;
K 2 Cr 2 O 7 + 14HCl \u003d 3Cl 2 + 2KCl + 2CrCl 3 + 7H 2 O;
2KMnO 4 +16HCl = 2MnCl 2 +5Cl 2 +8H 2 O +2KCl.
Application of halogens
Halogens are used as raw materials to produce various products. Thus, fluorine and chlorine are used for the synthesis of various polymer materials, chlorine is also a raw material in the production of hydrochloric acid. Bromine and iodine found wide application in medicine, bromine is also used by the paint and varnish industry.
Examples of problem solving
EXAMPLE 1
| Exercise | Calculate the volume of chlorine (no.) that reacted with potassium iodide if iodine weighing 508 g was formed | ||||
| Solution | Let us write the equation for the reaction between chlorine and potassium iodide: Cl 2 + 2KI = I 2 + 2KCl Molar mass of iodine, calculated using the table of chemical elements by D.I. Mendeleev, equal to – 254 g/mol. Let's find the amount of iodine formed: v(I 2) = m(I 2)/M(I 2) The halogens are located to the left of the noble gases in the periodic table. These five toxic non-metallic elements are in group 7 of the periodic table. These include fluorine, chlorine, bromine, iodine and astatine. Although astatine is radioactive and has only short-lived isotopes, it behaves like iodine and is often classified as a halogen. Since halogen elements have seven valence electrons, they only need one extra electron to form a full octet. This characteristic makes them more reactive than other groups of nonmetals. general characteristicsHalogens form diatomic molecules (type X2, where X denotes a halogen atom) - a stable form of existence of halogens in the form of free elements. The bonds of these diatomic molecules are non-polar, covalent and single. The chemical properties of halogens allow them to easily combine with most elements, so they are never found uncombined in nature. Fluorine is the most active halogen, and astatine is the least. All halogens form group I salts with similar properties. In these compounds, halogens are present as halide anions with a charge of -1 (for example, Cl-, Br-). The ending -id indicates the presence of halide anions; for example Cl- is called "chloride". Besides, Chemical properties halogens allow them to act as oxidizing agents - oxidizing metals. Majority chemical reactions, in which halogens participate - redox in an aqueous solution. Halogens form single bonds with carbon or nitrogen in organic compounds, where their oxidation state (CO) is -1. When a halogen atom is replaced by a covalently bonded hydrogen atom in organic compound, the prefix halo- can be used in a general sense, or the prefixes fluoro-, chloro-, bromo-, iodine- for specific halogens. Halogen elements can cross-link to form diatomic molecules with polar covalent single bonds. Chlorine (Cl2) was the first halogen discovered in 1774, followed by iodine (I2), bromine (Br2), fluorine (F2) and astatine (At, discovered last, in 1940). The name "halogen" comes from the Greek roots hal- ("salt") and -gen ("to form"). Together these words mean “salt-forming,” emphasizing the fact that halogens react with metals to form salts. Halite is the name for rock salt, a naturally occurring mineral composed of sodium chloride (NaCl). And finally, halogens are used in everyday life - fluoride is found in toothpaste, chlorine disinfects drinking water, and iodine promotes the production of thyroid hormones. Chemical elementsFluorine is an element with atomic number 9 and is designated by the symbol F. Elemental fluorine was first discovered in 1886 by isolating it from hydrofluoric acid. In its free state, fluorine exists as a diatomic molecule (F2) and is the most abundant halogen in the earth's crust. Fluorine is the most electronegative element on the periodic table. At room temperature it is a pale yellow gas. Fluorine also has a relatively small atomic radius. Its CO is -1, except in the elemental diatomic state, in which its oxidation state is zero. Fluorine is extremely reactive and reacts directly with all elements except helium (He), neon (Ne) and argon (Ar). In H2O solution, hydrofluoric acid (HF) is a weak acid. Although fluorine is highly electronegative, its electronegativity does not determine acidity; HF is a weak acid due to the fact that the fluoride ion is basic (pH > 7). In addition, fluorine produces very powerful oxidizing agents. For example, fluorine can react with inert gas xenon and forms a strong oxidizing agent xenon difluoride (XeF2). Fluoride has many uses. Chlorine is an element with atomic number 17 and the chemical symbol Cl. Discovered in 1774 by isolating it from hydrochloric acid. In its elemental state it forms the diatomic molecule Cl2. Chlorine has several COs: -1, +1, 3, 5 and 7. At room temperature it is a light green gas. Since the bond that forms between two chlorine atoms is weak, the Cl2 molecule has a very high ability to form compounds. Chlorine reacts with metals to form salts called chlorides. Chlorine ions are the most common ions found in seawater. Chlorine also has two isotopes: 35Cl and 37Cl. Sodium chloride is the most common compound of all the chlorides. Bromine – chemical element with atomic number 35 and symbol Br. It was first discovered in 1826. In its elemental form, bromine is a diatomic molecule Br2. At room temperature it is a reddish-brown liquid. Its COs are -1, + 1, 3, 4 and 5. Bromine is more active than iodine, but less active than chlorine. In addition, bromine has two isotopes: 79Br and 81Br. Bromine occurs as bromide salts dissolved in seawater. Behind last years The world's bromide production has increased significantly due to its availability and long shelf life. Like other halogens, bromine is an oxidizing agent and is very toxic. Iodine is a chemical element with atomic number 53 and symbol I. Iodine has oxidation states: -1, +1, +5 and +7. Exists as a diatomic molecule, I2. At room temperature it is solid purple. Iodine has one stable isotope - 127I. First discovered in 1811 using seaweed and sulfuric acid. Currently, iodine ions can be isolated in seawater. Although iodine is not very soluble in water, its solubility can be increased by using individual iodides. Iodine plays an important role in the body, participating in the production of thyroid hormones. Astatine is a radioactive element with atomic number 85 and the symbol At. Its possible oxidation states are -1, +1, 3, 5 and 7. The only halogen that is not a diatomic molecule. Under normal conditions it is a black metallic solid. Astatine is a very rare element, so little is known about it. In addition, astatine has very short period half-life, no longer than several hours. Obtained in 1940 as a result of synthesis. Astatine is believed to be similar to iodine. Differs in metallic properties. The table below shows the structure of halogen atoms and the structure of the outer layer of electrons. This structure of the outer layer of electrons means that the physical and chemical properties of halogens are similar. However, when comparing these elements, differences are also observed. Periodic properties in the halogen groupPhysical properties simple substances halogens change with increasing atomic number of the element. For better understanding and greater clarity, we offer you several tables. The melting and boiling points of a group increase as the molecular size increases (F Table 1. Halogens. Physical properties: melting and boiling points Kernel size increases (F< Cl < Br < I < At), так как увеличивается число протонов и нейтронов. Кроме того, с каждым периодом добавляется всё больше уровней энергии. Это приводит к большей орбитали, и, следовательно, к увеличению радиуса атома. Table 2. Halogens. Physical properties: atomic radii If the outer valence electrons are not located near the nucleus, then it will not take much energy to remove them from it. Thus, the energy required to eject an outer electron is not as high in the lower part of the element group, since there are more energy levels there. Additionally, high ionization energy causes the element to exhibit non-metallic qualities. Iodine and display astatine exhibit metallic properties because the ionization energy is reduced (At< I < Br < Cl < F). Table 3. Halogens. Physical properties: ionization energy The number of valence electrons in an atom increases with increasing energy levels at progressively lower levels. Electrons are progressively further away from the nucleus; Thus, the nucleus and electrons are not attracted to each other. An increase in shielding is observed. Therefore, Electronegativity decreases with increasing period (At< I < Br < Cl < F). Table 4. Halogens. Physical properties: electronegativity As atomic size increases with increasing period, electron affinity tends to decrease (B< I < Br < F < Cl). Исключение – фтор, сродство которого меньше, чем у хлора. Это можно объяснить меньшим размером фтора по сравнению с хлором. Table 5. Electron affinity of halogens The reactivity of halogens decreases with increasing period (At A halide is formed when a halogen reacts with another, less electronegative element to form a binary compound. Hydrogen reacts with halogens, forming halides of the form HX: Hydrogen halides easily dissolve in water to form hydrohalic acid (hydrofluoric, hydrochloric, hydrobromic, hydroiodic) acid. The properties of these acids are given below. Acids are formed by the following reaction: HX (aq) + H2O (l) → X- (aq) + H3O+ (aq). All hydrogen halides form strong acids, with the exception of HF. The acidity of hydrohalic acids increases: HF Hydrofluoric acid can etch glass and some inorganic fluorides for a long time. It may seem counterintuitive that HF is the weakest hydrohalic acid, since fluorine has the highest electronegativity. However, the H-F bond is very strong, resulting in a very weak acid. A strong bond is determined by a short bond length and high dissociation energy. Of all the hydrogen halides, HF has the shortest bond length and the highest bond dissociation energy. Halogen oxo acids are acids with hydrogen, oxygen and halogen atoms. Their acidity can be determined by structural analysis. The halogen oxo acids are given below: In each of these acids, a proton is bonded to an oxygen atom, so comparing proton bond lengths is not useful here. Electronegativity plays a dominant role here. Acid activity increases with the number of oxygen atoms associated with the central atom. The basic physical properties of halogens can be summarized in the following table. The color of halogens results from the absorption of visible light by molecules, which causes electrons to be excited. Fluorine absorbs violet light and therefore appears light yellow. Iodine, on the other hand, absorbs yellow light and appears violet (yellow and violet are complementary colors). The color of halogens becomes darker as the period increases. In closed containers, liquid bromine and solid iodine are in equilibrium with their vapors, which can be observed in the form of a colored gas. Although the color of astatine is unknown, it is assumed to be darker than iodine (i.e., black) according to the observed pattern. Now, if you are asked: “Characterize the physical properties of halogens,” you will have something to say. Oxidation number is often used instead of the concept of halogen valence. Typically, the oxidation state is -1. But if a halogen is bonded to oxygen or another halogen, it can take other states: oxygen CO -2 takes precedence. In the case of two different halogen atoms bonded together, the more electronegative atom prevails and accepts CO -1. For example, in iodine chloride (ICl), chlorine has CO -1, and iodine +1. Chlorine is more electronegative than iodine, so its CO is -1. In bromic acid (HBrO4), oxygen has CO -8 (-2 x 4 atoms = -8). Hydrogen has an overall oxidation state of +1. Adding these values gives an CO of -7. Since the final CO of the compound must be zero, the CO of bromine is +7. The third exception to the rule is the oxidation state of the halogen in elemental form (X2), where its CO is zero. Electronegativity increases with increasing period. Fluorine therefore has the highest electronegativity of all the elements, as evidenced by its position on the periodic table. Its electron configuration is 1s2 2s2 2p5. If fluorine gains another electron, the outermost p orbitals are completely filled and form a full octet. Since fluorine has high electronegativity, it can easily take an electron from a neighboring atom. Fluorine in this case is isoelectronic to the inert gas (with eight valence electrons), all its outer orbitals are filled. In this state, fluorine is much more stable. In nature, halogens are in the state of anions, so free halogens are obtained by oxidation by electrolysis or using oxidizing agents. For example, chlorine is produced by hydrolysis of a solution of table salt. The use of halogens and their compounds is diverse. DEFINITION Halogens– Group VIIA elements – fluorine (F), chlorine (Cl), bromine (Br) and iodine (I). Electronic configuration of the outer energy level of halogens ns 2 np 5. Since halogens lack only one electron before completing the energy level, in ORR they most often exhibit the properties of oxidizing agents. Oxidation states of halogens: from “-1” to “+7”. The only element of the halogen group, fluorine, exhibits only one oxidation state “-1” and is the most electronegative element. Halogen molecules are diatomic: F 2, Cl 2, Br 2, I 2. With increasing charge of the nucleus of an atom of a chemical element, i.e. when moving from fluorine to iodine, the oxidizing ability of halogens decreases, which is confirmed by the ability to displace lower halogens by higher ones from hydrohalic acids and their salts: Br 2 + 2HI = I 2 + 2HBr Cl 2 + 2KBr = Br 2 + 2KCl At no. fluorine is a light yellow gas with a pungent odor. Poisonous. Chlorine is a light green gas, just like fluorine, it has a pungent odor. Highly poisonous. At elevated pressure and room temperature it easily turns into a liquid state. Bromine is a heavy liquid of red-brown color with a characteristic unpleasant pungent odor. Liquid bromine, as well as its vapors, are highly toxic. Bromine is poorly soluble in water and well in non-polar solvents. Iodine is a dark gray solid with a metallic sheen. Iodine vapor is purple. Iodine easily sublimes, i.e. transforms into a gaseous state from a solid, while bypassing the liquid state. Halogens can be obtained by electrolysis of solutions or melts of halides: MgCl 2 = Mg + Cl 2 (melt) Most often, halogens are obtained by the oxidation reaction of hydrohalic acids: MnO 2 + 4HCl = MnCl 2 + Cl 2 +2H 2 O K 2 Cr 2 O 7 + 14HCl = 3Cl 2 + 2KCl +2CrCl 3 +7H 2 O 2KMnO 4 +16HCl = 2MnCl 2 +5Cl 2 +8H 2 O +2KCl Fluorine has the greatest chemical activity. Most chemical elements, even at room temperature, interact with fluorine, releasing a large amount of heat. Even water burns in fluorine: 2H 2 O + 2F 2 = 4HF + O 2 Free chlorine is less reactive than fluorine. It does not react directly with oxygen, nitrogen and noble gases. It interacts with all other substances like fluorine: 2Fe + Cl 2 = 2FeCl 3 2P + 5Cl 2 = 2PCl 5 When chlorine interacts with water in the cold, a reversible reaction occurs: Cl 2 + H 2 O↔HCl +HClO The mixture of reaction products is called chlorine water. When chlorine interacts with alkalis in the cold, mixtures of chlorides and hypochlorites are formed: Cl 2 + Ca(OH) 2 = Ca(Cl)OCl + H 2 O When chlorine is dissolved in a hot alkali solution, the following reaction occurs: 3Cl 2 + 6KOH=5KCl +KClO 3 +3H 2 O Bromine, like chlorine, dissolves in water and, partially reacting with it, forms the so-called “bromine water,” while iodine is practically insoluble in water. Iodine differs significantly in chemical activity from other halogens. It does not react with most non-metals, and reacts slowly with metals only when heated. The interaction of iodine with hydrogen occurs only with strong heating; the reaction is endothermic and highly reversible: H 2 + I 2 = 2HI - 53 kJ. EXAMPLE 1 Cl 2 + 2KI = I 2 + 2KCl Let's find the amount of iodine formed: v(I 2)=m(I 2)/M(I 2) v(I 2)=508/254=2 mol According to the reaction equation, the amount of chlorine substance. >> Chemistry: Production of halogens. Biological significance and application of halogens and their compounds Production of halogens
. Fluorine and chlorine are obtained by electrolysis of melts or solutions of their salts. For example, the process of electric | Trolysis of molten sodium chloride can be reflected by the equation: But if hydrogen can be obtained in other, more convenient and cheaper ways, for example from natural gas, then sodium hydroxide is obtained almost exclusively by electrolysis of a solution of table salt, like chlorine. Bromine and iodine are produced industrially by the reaction of their displacement from bromides and iodides, respectively. Biological significance of halogens.
Their application. You couldn't help but notice that the labels of many brands of toothpastes indicate the fluoride content in them - of course, not free fluoride, but its compounds. Thanks to this important component, which is involved in the construction of tooth enamel and bones, diseases such as dental caries are prevented. In addition, fluorine is a necessary element in metabolic processes in glands, muscles and nerve cells. Fluorine is also important in industrial production, where its main consumers are the nuclear industry and electrical engineering. Its compound Na3AlF6 (what is it called?) is used to produce aluminum. And in everyday life, Teflon cookware, named after fluorine-containing plastic - Teflon, is increasingly used. Chlorine- one of the chemical elements without which the existence of living organisms is unthinkable. Its basic form. entering the body is sodium chloride. It stimulates metabolism, hair growth, gives vigor and strength. Most NaCl is found in blood plasma. Hydrochloric acid HC1, which is part of gastric juice, plays a special role in digestion. Without 0.2% HCl, the process of food digestion practically stops. And although almost all food products contain some amount of table salt, a person is forced to add about 20 g of salt to food daily. In terms of industrial use, chlorine far exceeds all halogens. Chlorine and its compounds are necessary for bleaching linen and cotton fabrics, paper, etc. Particularly much of it is consumed in organic synthesis for the production of plastics, rubbers, dyes and solvents (Fig. 20). Many chlorine-containing compounds are used to control agricultural pests. Chlorine is consumed in large quantities to disinfect drinking water, although not without negative consequences (which substance is safer to use for this purpose?). In non-ferrous metallurgy, some metals (titanium, niobium, tantalum) are extracted from ores by chlorination. Chlorine has also found use for military purposes as a chemical warfare agent. Later it was replaced by other, more effective chlorine-containing toxic substances, such as phosgene COCl2. Chlorine is contained in a very dangerous substance for life and health - dioxin. Chlorine compounds are one of the reasons for the destruction of the Earth's ozone layer. Another halogen, bromine, is also very important for the human body. Compounds of this element regulate the processes of excitation and inhibition of the central nervous system, therefore, for the treatment of nervous diseases (insomnia, hysteria, neurasthenia, etc.), doctors prescribe “bromine” - bromine-containing drugs. Bromine is actively accumulated by some plants, including seaweed. It is in the sea that most of the bromine on our planet is concentrated, and the sea serves as the main supplier of bromine. It is estimated that about 4 million tons of bromine are released into the air every year along with seawater. It is clear that its content in the air of coastal areas is always higher than in areas far from the sea. This is one of the reasons why breathing sea air is so beneficial. Bromine, or more precisely, silver bromide AgBr, plays an equally important role in photography. Those who are involved in photography will probably immediately remember the names of types of photographic paper - “Uni-bromine”, “Bromine-portrait”, etc. Photographic paper contains a layer of silver bromide applied to it using gelatin, which easily decomposes under the influence of light: 2АgBr = 2Ag + Br2 And finally, the last of the halogens we are considering - iodine - is the element without which a person cannot live: its lack in water and food reduces the production of thyroid hormone and leads to endemic goiter. The regulating effects of thyroid hormone include muscle arousal, heartbeat, appetite, digestion, brain function and human temperament. Iodine enters the body with food: bread, eggs, milk, water, seaweed and with air (especially sea air) when breathing. An alcohol solution of iodine (5-10%), called iodine tincture, is used to treat wounds. Iodine is included in many medicines. The main consumers of iodine are the pharmaceutical and chemical industries, as well as the production of photosensitive photographic materials. 1. Production of halogens by electrolysis of melts and salt solutions. 2. Biological significance of halogens. 3. The use of chlorine and compounds of fluorine, chlorine and iodine. What volume of chlorine (no.) and what mass of sodium can be obtained by electrolysis of 585 g of sodium chloride containing 2% impurities? Calculate how many grams of a 40% alkali solution can be obtained from sodium, the mass of which you determined in the previous problem. The French chemist Scheele obtained chlorine from the reaction of manganese(IV) oxide with hydrochloric acid. As a result of this reaction, manganese(II) chloride and water are also formed. Write an equation for this reaction, consider redox processes and calculate the mass of manganese(IV) oxide and the amount of hydrogen chloride required to produce 100 liters of chlorine (n.o.), if its yield is 95% of the theoretically possible. Prepare a message about the positive and negative significance of halogens in human life. |
