Attachment reactions. Types of chemical reactions in organic chemistry Division of substituents in the benzene ring into two types
Read also
) bonds of another chemical compound. Accession can be carried out as a connection carbon-carbon, and by communication carbon heteroatom. Addition reactions are denoted by English letters "Ad".
General view of addition reactions by bond carbon-carbon:
General view of addition reactions by bond carbon-oxygen:
Usually, the reagent to which the addition occurs is called substrate, and the other ( "X-Y") - attack reagent.
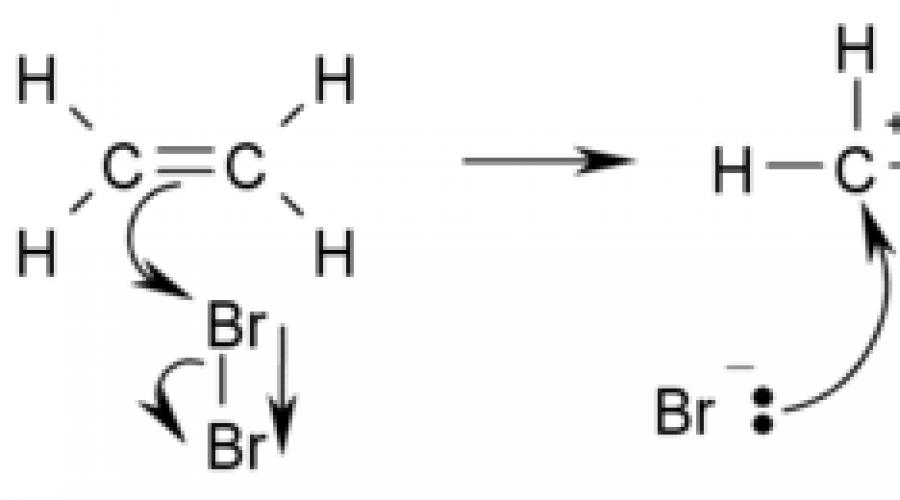
An example of an addition reaction is the bromination of ethylene:

Addition reactions are often reversible, pairing with elimination reactions, so it should be borne in mind that the mechanism for such a "paired" addition-elimination reaction is common.
Depending on the nature of the attacking particle and the reaction mechanism, a distinction is made between nucleophilic, electrophilic, radical, or synchronous addition.
Nucleophilic addition reactions
In nucleophilic addition reactions, the attacking particle is the nucleophile, that is, a negatively charged particle or a particle with a free electron pair.
General view of nucleophilic addition reactions:
Nucleophilic addition reactions are denoted "AdN".
Nucleophilic addition reactions at a bond C=C are quite rare, the most widespread and practical value is connection by connection C=O :
Among the reactions of nucleophilic addition, the most common is the above two-stage bimolecular mechanism Ad N 2: In the beginning, the nucleophile slowly adds at a multiple bond to form a carbanion, which in the second step is rapidly attacked by an electrophilic species.
Electrophilic addition reactions
In electrophilic addition reactions, the attacking particle is electrophile, that is, a positively charged particle, most often a proton H+, or an electron-deficient particle.
General view of electrophilic addition reactions:
Electrophilic addition reactions are denoted "Ad e".
Electrophilic addition reactions are widespread among the reactions of unsaturated hydrocarbons: alkenes, alkynes and dienes.
An example of such reactions is the hydration of alkenes:
Electrophilic bonding carbon heteroatom is also quite common, and most often such a connection is C=O:



Among the reactions of electrophilic addition, the most common is the above two-stage bimolecular mechanism Ad E 2: In the beginning, the electrophile slowly adds at a multiple bond to form a carbocation, which undergoes nucleophilic attack in the second step.
Radical addition reactions
In radical addition reactions, free radicals are the attacking species.
Radical addition reactions are denoted "Ad R".
Radical addition reactions usually proceed instead of electrophilic addition reactions in the presence of a source of free radicals:

Synchronous addition reactions
In some cases, addition by a multiple bond occurs with the simultaneous attack of both atoms, which does not allow one to determine the priority of the attack. Such a mechanism is called synchronous connection. Synchronous addition reactions lead to the formation of cyclic products, so they are often called cycloaddition.
Notes
| Chemical reactions in organic chemistry | |
|---|---|
| Substitution reactions | Nucleophilic substitution reactions Electrophilic substitution reactions Radical substitution reactions |
| Addition reactions | Nucleophilic addition reactions Electrophilic addition reactions Radical addition reactions Simultaneous addition reactions |
| Elimination reactions | Heterolytic elimination reactions Pericyclic elimination reactions Radical elimination reactions |
| rearrangement reactions | Nucleophilic rearrangements Electrophilic rearrangements Radical rearrangements |
| Oxidation and reduction reactions | Oxidation reactions Reduction reactions |
| Other | Nominal reactions in organic chemistry |
Wikimedia Foundation. 2010 .
See what "Addition reactions" are in other dictionaries:
addition reaction polymer- - EN addition polymer A polymer formed by the chain addition of unsaturated monomer molecules, such as olefins, with one another without the formation of a by product, as water;… … Technical Translator's Handbook
- (English addition electrophilic reaction) reactions of addition, in which the attack at the initial stage is carried out by an electrophile particle, positively charged or having a deficit of electrons. At the final stage, the resulting ... ... Wikipedia
- (English addition nucleophilic reaction) addition reactions in which the attack at the initial stage is carried out by a nucleophile particle, negatively charged or having a free electron pair. At the final stage, the resulting ... ... Wikipedia
- (English addition radical reaction) addition reactions in which the attack is carried out by free radicals of a particle containing one or more unpaired electrons. At the same time, radicals can attack both other radicals and ... ... Wikipedia
Addition reactions in which both atoms of a multiple bond are attacked simultaneously. Another name for reactions of this type is cycloaddition reactions, since the end products of such reactions are cyclic substrates. There are two ... ... Wikipedia
- (English nucleophilic substitution reaction) substitution reactions in which the attack is carried out by a nucleophile reagent carrying an unshared electron pair. The leaving group in nucleophilic substitution reactions is called a nucleofug. All ... Wikipedia
The reactions of organic substances can be formally divided into four main types: substitution, addition, elimination (elimination) and rearrangement (isomerization). Obviously, the whole variety of reactions of organic compounds cannot be reduced to the proposed classification (for example, combustion reactions). However, such a classification will help to establish analogies with the reactions already familiar to you that occur between inorganic substances.
As a rule, the main organic compound involved in the reaction is called substrate, and the other component of the reaction is conditionally considered as reagent.
Substitution reactions
Substitution reactions- these are reactions that result in the replacement of one atom or group of atoms in the original molecule (substrate) with other atoms or groups of atoms.
Substitution reactions involve saturated and aromatic compounds, such as alkanes, cycloalkanes, or arenes. Let us give examples of such reactions.
Under the action of light, hydrogen atoms in a methane molecule can be replaced by halogen atoms, for example, by chlorine atoms:
Another example of replacing hydrogen with halogen is the conversion of benzene to bromobenzene:

The equation for this reaction can be written differently:
![]()
With this form of writing, the reagents, catalyst, reaction conditions are written above the arrow, and the inorganic reaction products below it.
As a result of reactions substitutions in organic substances are formed not simple and complex substances, as in inorganic chemistry, and two complex substances.
Addition reactions
Addition reactions- These are reactions in which two or more molecules of reactants combine into one.
Unsaturated compounds, such as alkenes or alkynes, enter into addition reactions. Depending on which molecule acts as a reagent, hydrogenation (or reduction), halogenation, hydrohalogenation, hydration, and other addition reactions are distinguished. Each of them requires certain conditions.
1.Hydrogenation- the reaction of adding a hydrogen molecule to a multiple bond:
2. Hydrohalogenation- hydrogen halide addition reaction (hydrochlorination):
3. Halogenation- halogen addition reaction:
![]()
4.Polymerization- a special type of addition reactions, during which molecules of a substance with a small molecular weight are combined with each other to form molecules of a substance with a very high molecular weight - macromolecules.
Polymerization reactions are the processes of combining many molecules of a low molecular weight substance (monomer) into large molecules (macromolecules) of a polymer.
An example of a polymerization reaction is the production of polyethylene from ethylene (ethene) under the action of ultraviolet radiation and a radical polymerization initiator R.
The covalent bond most characteristic of organic compounds is formed when atomic orbitals overlap and the formation of common electron pairs. As a result of this, an orbital common to two atoms is formed, on which a common electron pair is located. When the bond is broken, the fate of these common electrons can be different.
Types of reactive particles
An orbital with an unpaired electron belonging to one atom may overlap with an orbital of another atom that also contains an unpaired electron. In this case, the formation of a covalent bond occurs according to the exchange mechanism:
The exchange mechanism for the formation of a covalent bond is realized if a common electron pair is formed from unpaired electrons belonging to different atoms.
The process opposite to the formation of a covalent bond by the exchange mechanism is bond breaking, in which one electron () goes to each atom. As a result, two uncharged particles with unpaired electrons are formed:
![]()
Such particles are called free radicals.
free radicals- atoms or groups of atoms having unpaired electrons.
Free radical reactions are reactions that occur under the action and with the participation of free radicals.
In the course of inorganic chemistry, these are reactions of interaction of hydrogen with oxygen, halogens, combustion reactions. Reactions of this type are characterized by high speed, release of a large amount of heat.
A covalent bond can also be formed by the donor-acceptor mechanism. One of the orbitals of an atom (or anion), which contains an unshared electron pair, overlaps with an unfilled orbital of another atom (or cation) that has an unfilled orbital, and a covalent bond is formed, for example:
![]()
Breaking a covalent bond leads to the formation of positively and negatively charged particles (); since in this case both electrons from a common electron pair remain with one of the atoms, the other atom gets an unfilled orbital:
![]()
Consider the electrolytic dissociation of acids:
![]()
One can easily guess that a particle having an unshared electron pair R: -, i.e., a negatively charged ion, will be attracted to positively charged atoms or to atoms on which there is at least a partial or effective positive charge.
Particles with unshared electron pairs are called nucleophilic agents (nucleus- "nucleus", the positively charged part of the atom), that is, the "friends" of the nucleus, a positive charge.
Nucleophiles(Nu) - anions or molecules that have a lone pair of electrons, interacting with the regions of the molecules, on which the effective positive charge is concentrated.
Examples of nucleophiles: Cl - (chloride ion), OH - (hydroxide anion), CH 3 O - (methoxide anion), CH 3 COO - (acetate anion).
Particles that have an unfilled orbital, on the contrary, will tend to fill it and, therefore, will be attracted to the regions of the molecules that have an increased electron density, a negative charge, and an unshared electron pair. They are electrophiles, “friends” of an electron, a negative charge, or particles with an increased electron density.
electrophiles- cations or molecules that have an unfilled electron orbital, tending to fill it with electrons, as this leads to a more favorable electronic configuration of the atom.
Not every particle is an electrophile with an empty orbital. So, for example, alkali metal cations have the configuration of inert gases and do not tend to acquire electrons, since they have a low electron affinity.
From this we can conclude that despite the presence of an unfilled orbital, such particles will not be electrophiles.
Main reaction mechanisms
There are three main types of reacting particles - free radicals, electrophiles, nucleophiles - and three corresponding types of reaction mechanism:
- free radical;
- electrophilic;
- nullophilic.
In addition to classifying reactions according to the type of reacting particles, organic chemistry distinguishes four types of reactions according to the principle of changing the composition of molecules: addition, substitution, elimination, or elimination (from the English. to eliminate- delete, split off) and regroup. Since addition and substitution can occur under the action of all three types of reactive species, several majorreaction mechanisms.


In addition, consider the cleavage or elimination reactions that take place under the influence of nucleophilic particles - bases.
6. Elimination:
A distinctive feature of alkenes (unsaturated hydrocarbons) is the ability to enter into addition reactions. Most of these reactions proceed by the mechanism of electrophilic addition.
Hydrohalogenation (addition of halogen hydrogen):
When a hydrogen halide is added to an alkene hydrogen is added to more hydrogenated carbon atom, i.e., the atom at which there are more atoms hydrogen, and halogen - to less hydrogenated.
Chemical properties of alkanes
Alkanes (paraffins) are non-cyclic hydrocarbons, in the molecules of which all carbon atoms are connected only by single bonds. In other words, there are no multiple, double or triple bonds in the molecules of alkanes. In fact, alkanes are hydrocarbons containing the maximum possible number of hydrogen atoms, and therefore they are called limiting (saturated).
Due to saturation, alkanes cannot enter into addition reactions.
Since carbon and hydrogen atoms have fairly close electronegativity, this leads to the fact that the CH bonds in their molecules are extremely low polarity. In this regard, for alkanes, reactions proceeding according to the mechanism of radical substitution, denoted by the symbol S R, are more characteristic.
1. Substitution reactions
In reactions of this type, carbon-hydrogen bonds are broken.
RH + XY → RX + HY
Halogenation
Alkanes react with halogens (chlorine and bromine) under the action of ultraviolet light or with strong heat. In this case, a mixture of halogen derivatives with different degrees of substitution of hydrogen atoms is formed - mono-, di-tri-, etc. halogen-substituted alkanes.
On the example of methane, it looks like this:
By changing the ratio of halogen/methane in the reaction mixture, it is possible to ensure that any particular halogen derivative of methane will predominate in the composition of the products.
reaction mechanism
Let us analyze the mechanism of the free radical substitution reaction using the example of the interaction of methane and chlorine. It consists of three stages:
- initiation (or chain initiation) - the process of formation of free radicals under the action of energy from the outside - irradiation with UV light or heating. At this stage, the chlorine molecule undergoes a homolytic cleavage of the Cl-Cl bond with the formation of free radicals:
Free radicals, as can be seen from the figure above, are called atoms or groups of atoms with one or more unpaired electrons (Cl, H, CH 3 , CH 2, etc.);
2. Chain development
This stage consists in the interaction of active free radicals with inactive molecules. In this case, new radicals are formed. In particular, when chlorine radicals act on alkane molecules, an alkyl radical and hydrogen chloride are formed. In turn, the alkyl radical, colliding with chlorine molecules, forms a chlorine derivative and a new chlorine radical:
3) Break (death) of the chain:
Occurs as a result of the recombination of two radicals with each other into inactive molecules:
2. Oxidation reactions
Under normal conditions, alkanes are inert with respect to such strong oxidizing agents as concentrated sulfuric and nitric acids, permanganate and potassium dichromate (KMnO 4, K 2 Cr 2 O 7).
Combustion in oxygen
A) complete combustion with an excess of oxygen. Leads to the formation of carbon dioxide and water:
CH 4 + 2O 2 \u003d CO 2 + 2H 2 O
B) incomplete combustion with a lack of oxygen:
2CH 4 + 3O 2 \u003d 2CO + 4H 2 O
CH 4 + O 2 \u003d C + 2H 2 O
Catalytic oxidation with oxygen
As a result of heating alkanes with oxygen (~200 o C) in the presence of catalysts, a wide variety of organic products can be obtained from them: aldehydes, ketones, alcohols, carboxylic acids.
For example, methane, depending on the nature of the catalyst, can be oxidized to methyl alcohol, formaldehyde, or formic acid:
3. Thermal transformations of alkanes
Cracking
Cracking (from the English to crack - to tear) is a chemical process occurring at high temperature, as a result of which the carbon skeleton of alkane molecules breaks with the formation of alkene and alkane molecules with lower molecular weights compared to the original alkanes. For example:
CH 3 -CH 2 -CH 2 -CH 2 -CH 2 -CH 2 -CH 3 → CH 3 -CH 2 -CH 2 -CH 3 + CH 3 -CH \u003d CH 2
Cracking can be thermal or catalytic. For the implementation of catalytic cracking, due to the use of catalysts, significantly lower temperatures are used compared to thermal cracking.
Dehydrogenation
The elimination of hydrogen occurs as a result of breaking the C-H bonds; carried out in the presence of catalysts at elevated temperatures. Dehydrogenation of methane produces acetylene:
2CH 4 → C 2 H 2 + 3H 2
Heating methane to 1200 ° C leads to its decomposition into simple substances:
CH 4 → C + 2H 2
Dehydrogenation of other alkanes gives alkenes:
C 2 H 6 → C 2 H 4 + H 2
When dehydrogenating n-butane, butene-1 and butene-2 are formed (the latter in the form cis- and trance-isomers):
Dehydrocyclization
Isomerization
Chemical properties of cycloalkanes
The chemical properties of cycloalkanes with more than four carbon atoms in the cycles are generally almost identical to those of alkanes. For cyclopropane and cyclobutane, oddly enough, addition reactions are characteristic. This is due to the high tension within the cycle, which leads to the fact that these cycles tend to break. So cyclopropane and cyclobutane easily add bromine, hydrogen or hydrogen chloride:
Chemical properties of alkenes
1. Addition reactions
Since the double bond in alkene molecules consists of one strong sigma bond and one weak pi bond, they are quite active compounds that easily enter into addition reactions. Alkenes often enter into such reactions even under mild conditions - in the cold, in aqueous solutions and organic solvents.
Hydrogenation of alkenes
Alkenes are able to add hydrogen in the presence of catalysts (platinum, palladium, nickel):
CH 3 -CH \u003d CH 2 + H 2 → CH 3 -CH 2 -CH 3
Hydrogenation of alkenes proceeds easily even at normal pressure and slight heating. An interesting fact is that the same catalysts can be used for the dehydrogenation of alkanes to alkenes, only the dehydrogenation process proceeds at a higher temperature and lower pressure.
Halogenation
Alkenes easily enter into an addition reaction with bromine both in aqueous solution and in organic solvents. As a result of the interaction, initially yellow solutions of bromine lose their color, i.e. discolor.
CH 2 \u003d CH 2 + Br 2 → CH 2 Br-CH 2 Br
Hydrohalogenation
It is easy to see that the addition of a hydrogen halide to an unsymmetrical alkene molecule should theoretically lead to a mixture of two isomers. For example, when hydrogen bromide is added to propene, the following products should be obtained:
Nevertheless, in the absence of specific conditions (for example, the presence of peroxides in the reaction mixture), the addition of a hydrogen halide molecule will occur strictly selectively in accordance with the Markovnikov rule:
The addition of a hydrogen halide to an alkene occurs in such a way that hydrogen is attached to a carbon atom with a large number of hydrogen atoms (more hydrogenated), and a halogen is attached to a carbon atom with a smaller number of hydrogen atoms (less hydrogenated).
Hydration
This reaction leads to the formation of alcohols, and also proceeds in accordance with the Markovnikov rule:
As you might guess, due to the fact that the addition of water to the alkene molecule occurs according to the Markovnikov rule, the formation of primary alcohol is possible only in the case of ethylene hydration:
CH 2 \u003d CH 2 + H 2 O → CH 3 -CH 2 -OH
It is by this reaction that the main amount of ethyl alcohol is carried out in the large-capacity industry.
Polymerization
A specific case of the addition reaction is the polymerization reaction, which, unlike halogenation, hydrohalogenation and hydration, proceeds through a free radical mechanism:
Oxidation reactions
Like all other hydrocarbons, alkenes burn easily in oxygen to form carbon dioxide and water. The equation for the combustion of alkenes in excess oxygen has the form:
C n H 2n + (3/2)nO 2 → nCO 2 + nH 2 O
Unlike alkanes, alkenes are easily oxidized. Under the action of an aqueous solution of KMnO 4 on alkenes, discoloration, which is a qualitative reaction to double and triple CC bonds in molecules of organic substances.
Oxidation of alkenes with potassium permanganate in a neutral or slightly alkaline solution leads to the formation of diols (dihydric alcohols):
C 2 H 4 + 2KMnO 4 + 2H 2 O → CH 2 OH–CH 2 OH + 2MnO 2 + 2KOH (cooling)
In an acidic environment, a complete cleavage of the double bond occurs with the transformation of the carbon atoms that formed the double bond into carboxyl groups:
5CH 3 CH=CHCH 2 CH 3 + 8KMnO 4 + 12H 2 SO 4 → 5CH 3 COOH + 5C 2 H 5 COOH + 8MnSO 4 + 4K 2 SO 4 + 17H 2 O (heating)
If the double C=C bond is at the end of the alkene molecule, then carbon dioxide is formed as a product of oxidation of the extreme carbon atom at the double bond. This is due to the fact that the intermediate oxidation product, formic acid, is easily oxidized by itself in an excess of an oxidizing agent:
5CH 3 CH=CH 2 + 10KMnO 4 + 15H 2 SO 4 → 5CH 3 COOH + 5CO 2 + 10MnSO 4 + 5K 2 SO 4 + 20H 2 O (heating)
In the oxidation of alkenes, in which the C atom at the double bond contains two hydrocarbon substituents, a ketone is formed. For example, the oxidation of 2-methylbutene-2 produces acetone and acetic acid.
The oxidation of alkenes, which breaks the carbon skeleton at the double bond, is used to establish their structure.
Chemical properties of alkadienes
Addition reactions
For example, the addition of halogens:
Bromine water becomes colorless.
Under normal conditions, the addition of halogen atoms occurs at the ends of the butadiene-1,3 molecule, while π bonds are broken, bromine atoms are attached to the extreme carbon atoms, and free valences form a new π bond. Thus, as if there is a "movement" of the double bond. With an excess of bromine, one more bromine molecule can be added at the site of the formed double bond.
polymerization reactions
Chemical properties of alkynes
Alkynes are unsaturated (unsaturated) hydrocarbons and therefore are capable of entering into addition reactions. Among the addition reactions for alkynes, electrophilic addition is the most common.
Halogenation
Since the triple bond of alkyne molecules consists of one stronger sigma bond and two weaker pi bonds, they are able to attach either one or two halogen molecules. The addition of two halogen molecules by one alkyne molecule proceeds by the electrophilic mechanism sequentially in two stages:
Hydrohalogenation
The addition of hydrogen halide molecules also proceeds by the electrophilic mechanism and in two stages. In both stages, the addition proceeds in accordance with the Markovnikov rule:
Hydration
The addition of water to alkynes occurs in the presence of ruthium salts in an acidic medium and is called the Kucherov reaction.
As a result of the hydration of the addition of water to acetylene, acetaldehyde (acetic aldehyde) is formed:
For acetylene homologues, the addition of water leads to the formation of ketones:
Alkyne hydrogenation
Alkynes react with hydrogen in two steps. Metals such as platinum, palladium, nickel are used as catalysts:
Alkyne trimerization
When acetylene is passed over activated carbon at high temperature, a mixture of various products is formed from it, the main of which is benzene, a product of acetylene trimerization:
Dimerization of alkynes
Acetylene also enters into a dimerization reaction. The process proceeds in the presence of copper salts as catalysts:
Alkyne oxidation
Alkynes burn in oxygen:
C n H 2n-2 + (3n-1) / 2 O 2 → nCO 2 + (n-1) H 2 O
The interaction of alkynes with bases
Alkynes with a triple C≡C at the end of the molecule, unlike other alkynes, are able to enter into reactions in which the hydrogen atom in the triple bond is replaced by a metal. For example, acetylene reacts with sodium amide in liquid ammonia:
HC≡CH + 2NaNH 2 → NaC≡CNa + 2NH 3,
and also with an ammonia solution of silver oxide, forming insoluble salt-like substances called acetylenides:
Thanks to this reaction, it is possible to recognize alkynes with a terminal triple bond, as well as to isolate such an alkyne from a mixture with other alkynes.
It should be noted that all silver and copper acetylenides are explosive substances.
Acetylides are able to react with halogen derivatives, which is used in the synthesis of more complex organic compounds with a triple bond:
CH 3 -C≡CH + NaNH 2 → CH 3 -C≡CNa + NH 3
CH 3 -C≡CNa + CH 3 Br → CH 3 -C≡C-CH 3 + NaBr
Chemical properties of aromatic hydrocarbons
The aromatic nature of the bond affects the chemical properties of benzenes and other aromatic hydrocarbons.
A single 6pi electron system is much more stable than conventional pi bonds. Therefore, for aromatic hydrocarbons, substitution reactions are more characteristic than addition reactions. Arenes enter into substitution reactions by an electrophilic mechanism.
Substitution reactions
Halogenation
Nitration
The nitration reaction proceeds best under the action of not pure nitric acid, but its mixture with concentrated sulfuric acid, the so-called nitrating mixture:
Alkylation
The reaction in which one of the hydrogen atoms at the aromatic nucleus is replaced by a hydrocarbon radical:
Alkenes can also be used instead of halogenated alkanes. Aluminum halides, ferric iron halides or inorganic acids can be used as catalysts.<
Addition reactions
hydrogenation
Accession of chlorine
It proceeds by a radical mechanism under intense irradiation with ultraviolet light:
Similarly, the reaction can proceed only with chlorine.
Oxidation reactions
Combustion
2C 6 H 6 + 15O 2 \u003d 12CO 2 + 6H 2 O + Q
incomplete oxidation
The benzene ring is resistant to oxidizing agents such as KMnO 4 and K 2 Cr 2 O 7 . The reaction does not go.
Division of substituents in the benzene ring into two types:
Consider the chemical properties of benzene homologues using toluene as an example.
Chemical properties of toluene
Halogenation
The toluene molecule can be considered as consisting of fragments of benzene and methane molecules. Therefore, it is logical to assume that the chemical properties of toluene should to some extent combine the chemical properties of these two substances taken separately. In particular, this is precisely what is observed during its halogenation. We already know that benzene enters into a substitution reaction with chlorine by an electrophilic mechanism, and catalysts (aluminum or ferric iron halides) must be used to carry out this reaction. At the same time, methane is also capable of reacting with chlorine, but by a free radical mechanism, which requires irradiation of the initial reaction mixture with UV light. Toluene, depending on the conditions under which it undergoes chlorination, is able to give either substitution products of hydrogen atoms in the benzene ring - for this you need to use the same conditions as in the chlorination of benzene, or substitution products of hydrogen atoms in the methyl radical, if on it, how to act on methane with chlorine when irradiated with ultraviolet radiation:
As you can see, the chlorination of toluene in the presence of aluminum chloride led to two different products - ortho- and para-chlorotoluene. This is due to the fact that the methyl radical is a substituent of the first kind.
If the chlorination of toluene in the presence of AlCl 3 is carried out in excess of chlorine, the formation of trichlorine-substituted toluene is possible:
Similarly, when toluene is chlorinated in the light at a higher chlorine / toluene ratio, dichloromethylbenzene or trichloromethylbenzene can be obtained:
Nitration
The substitution of hydrogen atoms for nitrogroup, during the nitration of toluene with a mixture of concentrated nitric and sulfuric acids, leads to substitution products in the aromatic nucleus, and not in the methyl radical:
Alkylation
As already mentioned, the methyl radical is an orientant of the first kind, therefore, its Friedel-Crafts alkylation leads to substitution products in the ortho and para positions:
Addition reactions
Toluene can be hydrogenated to methylcyclohexane using metal catalysts (Pt, Pd, Ni):
C 6 H 5 CH 3 + 9O 2 → 7CO 2 + 4H 2 O
incomplete oxidation
Under the action of such an oxidizing agent as an aqueous solution of potassium permanganate, the side chain undergoes oxidation. The aromatic nucleus cannot be oxidized under such conditions. In this case, depending on the pH of the solution, either a carboxylic acid or its salt will be formed.
; in this case, one p-bond is broken and one or two s-bonds are formed. To indicate connection reactions use the symbol Ad (from the English addition - joining); for p-tions of cycloaddition, such a symbol is not used.
depending on the nature substrate distinguish between connections reactions on isolated or coupled multiple bonds, for example: C=C, C=C, C=C-C=C, C=O, C=N, C=N. Distinguish p-tion homolytic. (Ad R) and heterolytic. accessions. The latter depending on the charge of the attacker reagent subdivided into r-tion elektrof. (Ad E) and nucleoph. (AdN)additions. Behavior reagent depends on the type substrate and the conditions for conducting the distribution (distribution, the presence catalyst, the effect of UV irradiation, etc.). Mn. reagents in different conditions can show different. types of reactions. abilities, eg. halogens can act as radical, elektrof. and even the nucleoph. agents.
Naib. accessions studied reactions on multiple bonds carbon-carbon. These processes proceed according to a stepwise (staged) or synchronous (coordinated) mechanism. With a stepwise mechanism, the first stage is the attack of the nucleophile, electrophile, or free. radical, the second - recombination resulting intermediate positive, negative or a neutral particle, for example:
Electrof. or nucleoph. particles do not have to be ions; they can represent an electron-withdrawing or electron-donating part (group) molecules. R-tions of Ad N are possible only with C=C bonds activated by electron-withdrawing substituents; the implementation of Ad E requires either unsubstituted C=C bonds or those activated by electron-donating substituents. For p-tion Ad R, the nature of the substituent in the C=C bond does not matter much.
Stereochem. the result of the step addition depends on the p-tion mechanism and the nature of the reacting compounds. Yes, electro. joining olefins can proceed as son-attachment-particles Y and W attack molecule on one side of the plane double bond or as anti-attachment - particles attack from different sides of the plane; in some cases, districts go non-stereospecifically. Nucleof. joining with carbanions proceeds, as a rule, non-stereospecifically. When joining reactions on triple bonds syn addition leads to the cis isomer, anti addition leads to the trans isomer.
In the case of a synchronous mechanism, an attack on both atom C is carried out simultaneously and the p-tion proceeds as a dipolar addition (see Cycloaddition), while the addition reactions by double or triple bond go as son-attachment (see, e.g., Reppe reactions).
P connections reactions by conjugate double bonds, flowing through a stepwise mechanism, lead to the formation of 1,2- and 1,4-addition products:
Synchronous 1,4 connection to dienes trail is running. way:

A special type of addition reactions is conjugated addition. The flow of such p-tions is accompanied by the binding of the p-solvent (or specially added reagent) at the final stage of the process. For example, conjugated elektrof. accession halogens to alkenes in CH 3 COOH leads, along with 1,2-dihalides, to b-acetoxyalkyl halides:
Examples of conjugated nucleoph. accession - Michael reaction and interaction. activated alkenes with cyanide anion in proton p-solvents SH:

In case of joining reactions on multiple bonds carbon-hetero-atom, in which put. charge is localized atom C (bonds C=O, C=N, C=N and C=S), nucleophiles always attach to atom C, and electrophiles to a heteroatom. In naib. degree studied nucleophilic additions reactions for the carbonyl group:

P connections reaction on atom C may be one of the stages of p-tion substitution in aromatic. in a row, for example: