Amazing discoveries of quantum physics. Physicists have found a way to see the “smile” of quantum gravity. What experimental dependence contributed to the emergence of quantum gravity?
Read also
Nobody in the world understands quantum mechanics - this is the main thing you need to know about it. Yes, many physicists have learned to use its laws and even predict phenomena using quantum calculations. But it is still not clear why the presence of an observer determines the fate of the system and forces it to make a choice in favor of one state. “Theories and Practices” selected examples of experiments, the outcome of which is inevitably influenced by the observer, and tried to figure out what quantum mechanics is going to do with such interference of consciousness in material reality.
Shroedinger `s cat
Today there are many interpretations of quantum mechanics, the most popular of which remains the Copenhagen one. Its main principles were formulated in the 1920s by Niels Bohr and Werner Heisenberg. And the central term of the Copenhagen interpretation was the wave function - a mathematical function that contains information about all possible states of a quantum system in which it simultaneously resides.
According to the Copenhagen interpretation, only observation can reliably determine the state of a system and distinguish it from the rest (the wave function only helps to mathematically calculate the probability of detecting a system in a particular state). We can say that after observation, a quantum system becomes classical: it instantly ceases to coexist in many states at once in favor of one of them.
This approach has always had its opponents (remember, for example, “God doesn’t play dice” by Albert Einstein), but the accuracy of calculations and predictions has taken its toll. However, recently there have been fewer and fewer supporters of the Copenhagen interpretation, and not the least reason for this is the very mysterious instantaneous collapse of the wave function during measurement. Erwin Schrödinger's famous thought experiment with the poor cat was precisely intended to show the absurdity of this phenomenon.
So, let us recall the contents of the experiment. A live cat, an ampoule with poison and a certain mechanism that can at random put the poison into action are placed in a black box. For example, one radioactive atom, the decay of which will break the ampoule. The exact time of atomic decay is unknown. Only the half-life is known: the time during which decay will occur with a 50% probability.
It turns out that for an external observer, the cat inside the box exists in two states at once: it is either alive, if everything goes fine, or dead, if decay has occurred and the ampoule has broken. Both of these states are described by the cat's wave function, which changes over time: the further away, the greater the likelihood that radioactive decay has already occurred. But as soon as the box is opened, the wave function collapses and we immediately see the outcome of the knacker’s experiment.
It turns out that until the observer opens the box, the cat will forever balance on the border between life and death, and only the action of the observer will determine its fate. This is the absurdity that Schrödinger pointed out.
Electron diffraction

According to a survey of leading physicists conducted by The New York Times, the experiment with electron diffraction, carried out in 1961 by Klaus Jenson, became one of the most beautiful in the history of science. What is its essence?
There is a source emitting a flow of electrons towards the photographic plate screen. And there is an obstacle in the way of these electrons - a copper plate with two slits. What kind of picture can you expect on the screen if you think of electrons as just small charged balls? Two illuminated stripes opposite the slits.
In reality, a much more complex pattern of alternating black and white stripes appears on the screen. The fact is that when passing through the slits, electrons begin to behave not like particles, but like waves (just as photons, particles of light, can simultaneously be waves). Then these waves interact in space, weakening and strengthening each other in some places, and as a result a complex picture of alternating light and dark stripes appears on the screen.
In this case, the result of the experiment does not change, and if electrons are sent through the slit not in a continuous flow, but individually, even one particle can simultaneously be a wave. Even one electron can simultaneously pass through two slits (and this is another important position of the Copenhagen interpretation of quantum mechanics - objects can simultaneously exhibit their “usual” material properties and exotic wave properties).
But what does the observer have to do with it? Despite the fact that his already complicated story became even more complicated. When, in similar experiments, physicists tried to detect with the help of instruments which slit the electron actually passed through, the picture on the screen changed dramatically and became “classical”: two illuminated areas opposite the slits and no alternating stripes.
It was as if the electrons did not want to show their wave nature under the watchful gaze of the observer. We adjusted to his instinctive desire to see a simple and understandable picture. Mystic? There is a much simpler explanation: no observation of the system can be carried out without physical influence on it. But we’ll come back to this a little later.
Heated fullerene

Experiments on particle diffraction were carried out not only on electrons, but also on much larger objects. For example, fullerenes are large, closed molecules made up of dozens of carbon atoms (for example, a fullerene of sixty carbon atoms is very similar in shape to a soccer ball: a hollow sphere stitched together from pentagons and hexagons).
Recently, a group from the University of Vienna, led by Professor Zeilinger, tried to introduce an element of observation into such experiments. To do this, they irradiated moving fullerene molecules with a laser beam. Afterwards, heated by external influence, the molecules began to glow and thereby inevitably revealed to the observer their place in space.
Along with this innovation, the behavior of molecules also changed. Before the start of total surveillance, fullerenes quite successfully skirted obstacles (exhibited wave properties) like electrons from the previous example passing through an opaque screen. But later, with the appearance of an observer, fullerenes calmed down and began to behave like completely law-abiding particles of matter.
Cooling dimension

One of the most famous laws of the quantum world is Heisenberg's uncertainty principle: it is impossible to simultaneously determine the position and speed of a quantum object. The more accurately we measure the momentum of a particle, the less accurately its position can be measured. But the effects of quantum laws operating at the level of tiny particles are usually unnoticeable in our world of large macro objects.
Therefore, the more valuable are the recent experiments of Professor Schwab’s group from the USA, in which quantum effects were demonstrated not at the level of the same electrons or fullerene molecules (their characteristic diameter is about 1 nm), but on a slightly more tangible object - a tiny aluminum strip.
This strip was secured on both sides so that its middle was suspended and could vibrate under external influence. In addition, next to the strip there was a device capable of recording its position with high accuracy.
As a result, the experimenters discovered two interesting effects. Firstly, any measurement of the object’s position or observation of the strip did not pass without leaving a trace for her - after each measurement the position of the strip changed. Roughly speaking, experimenters determined the coordinates of the strip with great accuracy and thereby, according to the Heisenberg principle, changed its speed, and therefore its subsequent position.
Secondly, and quite unexpectedly, some measurements also led to the cooling of the strip. It turns out that an observer can change the physical characteristics of objects just by his presence. It sounds completely incredible, but to the credit of physicists, let’s say that they were not at a loss - now Professor Schwab’s group is thinking about how to apply the discovered effect to cool electronic chips.
Freezing particles

As you know, unstable radioactive particles decay in the world not only for the sake of experiments on cats, but also completely on their own. Moreover, each particle is characterized by an average lifetime, which, it turns out, can increase under the watchful gaze of the observer.
This quantum effect was first predicted back in the 1960s, and its brilliant experimental confirmation appeared in a paper published in 2006 by the group of Nobel laureate physicist Wolfgang Ketterle at the Massachusetts Institute of Technology.
In this work, we studied the decay of unstable excited rubidium atoms (decay into rubidium atoms in the ground state and photons). Immediately after the system was prepared and the atoms were excited, they began to be observed - they were illuminated with a laser beam. In this case, the observation was carried out in two modes: continuous (small light pulses are constantly supplied to the system) and pulsed (the system is irradiated from time to time with more powerful pulses).
The results obtained were in excellent agreement with theoretical predictions. External light influences actually slow down the decay of particles, as if returning them to their original state, far from decay. Moreover, the magnitude of the effect for the two regimes studied also coincides with predictions. And the maximum life of unstable excited rubidium atoms was extended by 30 times.
Quantum mechanics and consciousness
Electrons and fullerenes cease to exhibit their wave properties, aluminum plates cool, and unstable particles freeze in their decay: under the omnipotent gaze of the observer, the world is changing. What is not evidence of the involvement of our mind in the work of the world around us? So maybe Carl Jung and Wolfgang Pauli (Austrian physicist, Nobel Prize laureate, one of the pioneers of quantum mechanics) were right when they said that the laws of physics and consciousness should be considered complementary?
But this is only one step away from the routine recognition: the whole world around us is the essence of our mind. Creepy? (“Do you really think that the Moon exists only when you look at it?” Einstein commented on the principles of quantum mechanics). Then let's try to turn to physicists again. Moreover, in recent years they have become less and less fond of the Copenhagen interpretation of quantum mechanics with its mysterious collapse of a function wave, which is being replaced by another, quite down-to-earth and reliable term - decoherence.
The point is this: in all the observational experiments described, the experimenters inevitably influenced the system. They illuminated it with a laser and installed measuring instruments. And this is a general, very important principle: you cannot observe a system, measure its properties without interacting with it. And where there is interaction, there is a change in properties. Moreover, when the colossus of quantum objects interacts with a tiny quantum system. So eternal, Buddhist neutrality of the observer is impossible.
This is precisely what explains the term “decoherence” - an irreversible process of violation of the quantum properties of a system during its interaction with another, larger system. During such interaction, the quantum system loses its original features and becomes classical, “submitting” to the large system. This explains the paradox with Schrödinger's cat: the cat is such a large system that it simply cannot be isolated from the world. The thought experiment itself is not entirely correct.
In any case, compared to reality as an act of creation of consciousness, decoherence sounds much calmer. Maybe even too calm. After all, with this approach, the entire classical world becomes one big decoherence effect. And according to the authors of one of the most serious books in this field, statements like “there are no particles in the world” or “there is no time at a fundamental level” also logically follow from such approaches.
Creative observer or all-powerful decoherence? You have to choose between two evils. But remember - now scientists are increasingly convinced that the basis of our thought processes are those same notorious quantum effects. So where observation ends and reality begins - each of us has to choose.
VKontakte Facebook Odnoklassniki
When high-energy particles interact at a collider, a huge number of different particles are formed
This process is called multiple production, and its various characteristics are predicted using the theory of strong interactions - quantum chromodynamics (QCD). However, the results of recent similar experiments at the LHC (Large Hadron Collider) do not coincide with the predictions of models built from the results of past experiments at other accelerators. Nick Brooke, a professor at the University of Bristol and one of the leading experts in the field of studying multiple particle production, spoke at the Ginzburg Conference about the possible reasons for this discrepancy and the opening horizons of new experimental high-energy physics.
The technique of two experimental projects taking place at the LHC is ideal for identifying born particles. These are the ALICE project (A Large Ion Collider Experiment), optimized for studying collisions of heavy ions, and LHCb, designed to study B-mesons - particles containing a “pretty” quark. And the information about the birth of particles itself is a necessary foundation for the further development of QCD. Nick Brooke comments: “The observed particle distributions characterize the hadronic state of matter and are sensitive to the underlying quantum chromodynamics of proton-proton interactions. ALICE, ATLAS and CMS have already measured particle distributions in the central interaction region, and the geometry of LHCb allows us to track collision dynamics in the distant region. This gives us much-needed information to develop models and improve Monte Carlo event generators.”
Quantum chromodynamics arose in the 70s of the last century as a microscopic theory that describes the strong interaction on subhadron scales, which involves quarks, gluons and particles composed of them - hadrons, including protons and neutrons of the atomic nucleus bound by strong interaction. The basic postulate of quantum chromodynamics assigns to all quarks a special quantum number, called a color charge or color. Such a familiar word has nothing to do with ordinary optical characteristics, but it succinctly emphasizes the fact that in nature quarks are found only in the form of colorless combinations - hadrons, made up of three quarks (remember the analogy: red, green and blue add up to white) , or gluons from a quark and an antiquark with an anticolor.
QCD predictions about the parameters of multiple particle production are given either in analytical form or in the form of numerical computer calculations using Monte Carlo models, which can be compared in detail with experimental data. These models are called event generators in the sense that the probability of occurrence of certain phenomena in these computer calculations is considered to be proportional to the probability of the corresponding event in the real world. All of these models worked well in agreement with past experiments at other accelerators and even had some predictive power, but they do not yet coincide with the new results obtained at the LHC.
FIAN professor and leading researcher in the high-energy physics sector Andrei Leonidov comments: “The study of multiple production at high energies is one of the fundamental physical problems, and Brook’s report was dedicated to the array of experimental information that was accumulated at the LHC collider. A very interesting situation has arisen there: the existing models do not describe many essential properties of events. Their typical design somehow combines the physics of soft hadronic jets and hard hadronic radiation, and they themselves were calibrated to successfully describe FNAL, the previous accelerator. As a result, there was literally not a single graph in this report in which the theory coincided with the new experiment. That is, modern models do not describe many properties of multiple births at all.”
Thus, Professor Brook spoke about discrepancies between predictions and real data on the emergence of particles with “strange” quarks in their composition or violations in the ratio of baryonic and antibaryon matter. But all these inconsistencies, as Brook emphasized, only give researchers a free hand and once again show the complex structure of QCD. After all, new data can help improve models of event generators, soft particle production, multiparticle collisions and many other phenomena.
Andrei Leonidov also agrees with the optimism of the English physicist: “All previous models in new experiments have shown themselves to be unsuccessful to varying degrees, and this creates an interesting field for study. But these same models were put together for a reason: this is the best that humanity can offer on this topic. It’s not like some provincial people wrote something there, and it is accidentally used at the LHC. The LHC uses the best that is available, and this best is not working well yet. And this topic is very important, because multiple birth processes constantly occur in the collider. These are dominant processes with a large cross-section, and they potentially influence all other processes and determine their background. Moreover, it is fundamental and interesting. So there is nothing sad, we are waiting for new results!”

When high-energy particles collide, multiple creation of new particles is observed
“Anyone who wasn’t shocked when they first encountered quantum theory probably just didn’t understand.” Niels Bohr
The premises of quantum theory are so stunning that it looks more like science fiction.
A particle of the microworld can be in two or more places at the same time!
(One very recent experiment showed that one of these particles can be in 3000 places at the same time!)
The same “object” can be both a localized particle and an energy wave propagating in space.
Einstein postulated that nothing can travel faster than the speed of light. But quantum physics has proven: subatomic particles can exchange information instantly - located at any distance from each other.
Classical physics was deterministic: given initial conditions, such as the location and speed of an object, we can calculate where it will go. Quantum physics is probabilistic: we can never say with absolute certainty how the object under study will behave.
Classical physics was mechanistic. It is based on the premise that only by knowing the individual parts of an object can we ultimately understand what it is.
Quantum physics is holistic: it paints a picture of the Universe as a single whole, the parts of which are interconnected and influence each other.
And perhaps most importantly, quantum physics destroyed the idea of a fundamental difference between subject or object, observer and observed - which had dominated scientific minds for 400 years!
In quart physics, the observer influences the observed object. There are no isolated observers of the mechanical Universe - everything takes part in its existence.
SHOCK #1 - EMPTY SPACE
One of the first cracks in the solid structure of Newtonian physics was made by the following discovery: atoms are the solid building blocks of the physical Universe! - consist mainly of empty space. How empty? If you enlarge the nucleus of a hydrogen atom to the size of a basketball, the only electron orbiting it would be thirty kilometers away, with nothing between the nucleus and the electron. So, as you look around, remember: reality is the smallest points of matter surrounded by emptiness.
However, this is not entirely true. This supposed "emptiness" is not actually empty: it contains a colossal amount of incredibly powerful energy. We know that energy becomes more dense as it moves to a lower level of matter (for example, nuclear energy is a million times more powerful than chemical energy). Scientists now say that there is more energy in one cubic centimeter of empty space than in all the matter in the known universe. Although scientists have not been able to measure it, they are seeing the results of this sea of energy.
SHOCK #2 - PARTICLE, WAVE OR WAVEPARTICLE?
Not only is the atom almost entirely made up of “space,” but when scientists examined it more deeply, they discovered that the subatomic (constituting the atom) particles are not solid either. And they seem to have a dual nature. Depending on how we observe them, they can behave either like solid microbodies or like waves.
Particles are individual solid objects that occupy a certain position in space. But waves do not have a “body”; they are not localized and propagate in space.
As a wave, an electron or photon (particle of light) does not have a precise location, but exists as a “field of probabilities.” In the particle state, the probability field “collapses” (collapses) into a solid object. Its coordinates in four-dimensional space-time can already be determined.
This is surprising, but the state of a particle (wave or solid object) is determined by acts of observation and measurement. Unmeasured and unobservable electrons behave like waves. As soon as we subject them to observation during the experiment, they “collapse” into solid particles and can be recorded in space.
But how can something be both a solid particle and a fluid wave at the same time? Perhaps the paradox will be resolved if we remember what we said recently: particles behave like waves or like solid objects. But the concepts of "wave" and "particle" are just analogies taken from our everyday world. The concept of a wave was introduced into quantum theory by Erwin Schrödinger. He is the author of the famous “wave equation,” which mathematically substantiates the existence of wave properties in a solid particle before the act of observation. Some physicists - in an attempt to explain something they have never encountered and cannot fully understand - call subatomic particles "wave particles."
SHOCK #3 - QUANTUM LEAPS AND PROBABILITY
While studying the atom, scientists discovered that when electrons, rotating around the nucleus, move from orbit to orbit, they do not move through space like ordinary objects. No, they cover the distance instantly. That is, they disappear in one place and appear in another. This phenomenon was called a quantum leap.
Moreover, scientists realized that they could not determine exactly where in the new orbit the missing electron would appear or at what moment it would make a jump. The most they could do was calculate the probability (based on the Schrödinger wave equation) of the electron's new location.
“Reality, as we experience it, is created at each moment in the totality of countless possibilities,” says Dr. Satinover. “But the real secret is that there is nothing in the physical Universe that determines which possibility from this totality will come true. There is no process that establishes that.”
Thus, quantum leaps are the only truly random events in the Universe.
SHOCK #4 - THE PRINCIPLE OF UNCERTAINTY
In classical physics, all parameters of an object, including its spatial coordinates and speed, can be measured with an accuracy limited only by the capabilities of experimental technologies. But at the quantum level, whenever you determine one quantitative characteristic of an object, such as speed, you cannot obtain precise values for its other parameters, such as coordinates. In other words: if you know how fast an object is moving, you cannot know where it is. And vice versa: if you know where it is, you cannot know how fast it is moving.
No matter how sophisticated the experimenters are, no matter how advanced measurement technologies they use, they are unable to look behind this veil.
Werner Heisenberg, one of the pioneers of quantum physics, formulated the uncertainty principle. Its essence is this: no matter how hard you try, it is simultaneously impossible to obtain exact values of the coordinates and speed of a quantum object. The more precision we achieve in measuring one parameter, the more uncertain the other becomes.
SHOCK #5 - NONLOCALITY, EPR PARADOX AND BELL'S THEOREM
Albert Einstein did not like quantum physics. Assessing the probabilistic nature of subatomic processes outlined in quantum physics, he said: “God does not play dice with the Universe.” But Niels Bohr answered him: “Stop teaching God what to do!”
In 1935, Einstein and his colleagues Podolsky and Rosen (EPR) attempted to defeat quantum theory. Scientists, based on the principles of quantum mechanics, conducted a thought experiment and came to a paradoxical conclusion. (He was supposed to show the inferiority of quantum theory). The essence of their thoughts is this. If we have two simultaneously arising particles, this means that they are interconnected or are in a state of superposition. Let's send them to different ends of the Universe. Then we change the state of one of the particles. Then, according to quantum theory, another particle instantly comes to the same state. Instantly! On the other edge of the universe!
Such an idea was so ridiculous that Einstein sarcastically referred to it as “supernatural action at a distance.” According to his theory of relativity, nothing can travel faster than light. And in the EPR experiment it turned out that the speed of information exchange between particles is infinite! In addition, the very idea that an electron could “track” the state of another electron on the opposite edge of the Universe completely contradicted generally accepted ideas about reality, and indeed common sense in general.
But in 1964, the Irish theoretical physicist John Bell formulated and proved a theorem from which it followed: the “ridiculous” conclusions from the EPR thought experiment are true!
Particles are intimately connected on a level that transcends time and space. Therefore, they are able to instantly exchange information.
The idea that any object in the Universe is local - i.e. exists in one place (point) in space - not true. Everything in this world is non-local.
Nevertheless, this phenomenon is a valid law of the Universe. Schrödinger said that the relationship between objects is not the only interesting aspect of quantum theory, but it is the most important. In 1975, theoretical physicist Henry Stapp called Bell's theorem "the most significant discovery of science." Note that he was talking about science, not just physics.
(The article was prepared based on the materials of the book by W. Arntz, B. Chace, M. Vicente “The Rabbit Hole, or what do we know about ourselves and the Universe?”, chapter “Quantum Physics”.)
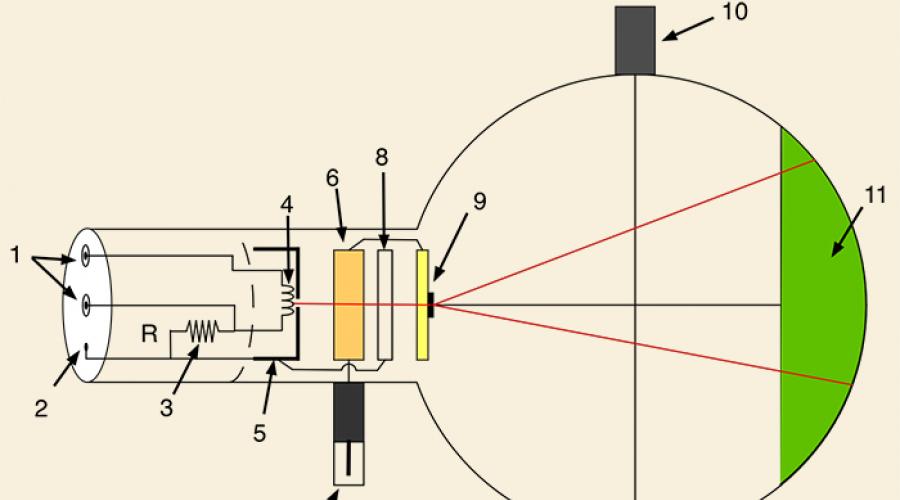
The birth of quantum theory. Photo effect.
Lesson objectives:
1. Consider the phenomenon of the photoelectric effect and study its laws
2. Develop logic, the ability to work in pairs; learn to simulate processes on a computer.
3. To develop the cognitive activity of schoolchildren with the help of historical material.
Equipment for the lesson: interactive whiteboard, computers on student desks, projector, speakers, digital center kit s ch oo l - collection . edu. ru
During the classes:
1. Prerequisites for the creation of quantum theory. (Teacher's story)
At the end of the 19th century. Many scientists believed that the development of physics was completed for the following reasons:
1. The laws of mechanics and the theory of universal gravitation have existed for more than 200 years.
2. Developed by MKT.
3. Podve provides a solid foundation for thermodynamics.
4. Maxwell's theory of electromagnetism is completed.
5. Fundamental laws of conservation (energy, momentum, angular momentum, mass and electric charge) were discovered.
At the end of the XIX - beginning of the 20th century discovered by V. Roentgen - X- rays (X-rays), A. Becquerel - the phenomenon of radioactivity, J. Thomson - electron. However, classical physics was unable to explain these phenomena.
A. Einstein's theory of relativity required a radical revision of the concept of space and time. Special experiments confirmed the validity of J. Maxwell's hypothesis about the electromagnetic nature of light. It could be assumed that the emission of electromagnetic waves by heated bodies is due to the oscillatory motion of electrons. But this assumption had to be confirmed by comparing theoretical and experimental data.
For theoretical consideration of the laws of radiation we used black body model , i.e. a body that completely absorbs electromagnetic waves of any length and, accordingly, emits all lengths of electromagnetic waves.
An example of an absolutely black body in terms of emissivity would be the Sun, and in terms of absorption - a cavity with mirror walls with a small hole.
The English physicist J. Rayleigh attempted a more rigorous theoretical derivation of the law of energy distribution. The law led to good agreement with experiments in the field of low frequencies. According to this law, the radiation intensity should increase in proportion to the square of the frequency. Consequently, thermal radiation should contain many ultraviolet and x-rays, which was not observed experimentally. Difficulties in reconciling theory with experimental results are called ultraviolet disaster.
The laws of electromagnetism obtained by Maxwell were unable to explain the shape of the intensity distribution curve in the spectrum of an absolutely black body. As you move away from this value, the intensity of electromagnetic radiation gradually decreases.
Trying to overcome the difficulties of classical theory in explaining black body radiation, M. Planck in 1900 Mr. made a hypothesis: atoms emit electromagnetic energy in separate portions — quanta . Energy E each portion is directly proportional to the radiation frequency:
Thus, M. Planck showed the way out of the difficulties encountered by the theory of thermal radiation, after which the modern physical theory called quantum physics .
2 . Concept of photoelectric effect
In the development of quantum theory, an important step was made in the study of one remarkable phenomenon discovered by G. Hertz and carefully studied by the Russian physicist A.G. Stoletov. This phenomenon is called the photoelectric effect.
A video is watched, after which students define the photoelectric effect.
As a result of research, three laws of the photoelectric effect were established.
1. The strength of the saturation current is directly proportional to the intensity of light radiation incident on the surface of the body.
2. The maximum kinetic energy of photoelectrons increases linearly with the frequency of light and depends on its intensity.
3. If the frequency of light is less than a certain minimum frequency determined for a given substance, then the photoelectric effect does not occur.
The dependence of photocurrent on voltage is shown in the figure.
3. Theory of the photoelectric effect.
The theory of the photoelectric effect was created by the German scientist A. Einstein in 1905. Einstein’s theory is based on the concept of the work function of electrons from a metal and the concept of quantum radiation of light. According to Einstein's theory, the photoelectric effect has the following explanation: by absorbing a quantum of light, an electron acquires energy. This energy is used to perform the work function and impart kinetic energy to the electron.
hOS=APIых+mv22">
hν - photon energy, which goes to the work function A of the electron from the metal and imparting kinetic energy to it.
Work function is the minimum work that must be done to remove an electron from a substance.
Einstein was awarded the Nobel Prize for his equation for the photoelectric effect in 1921.
Quantum theory provides the following explanations for the laws of the photoelectric effect.
As the intensity of monochromatic radiation increases, the number of quanta absorbed by the metal increases, and, consequently, the number of electrons emitted from it increases, therefore the photocurrent is directly proportional to the radiation intensity (1st law).
- 97.50 KbMinistry of Education and Science of the Russian Federation
Federal State Educational Institution of Secondary Professional Education "Alekseevsky College of Economics and Information Technologies"
"The emergence and development of quantum physics"
Completed by: student of group 22
specialties: 080110
Economics and Accounting
(by industry)
Rysikov Artem
Checked by: general education teacher
Koryaka Lyudmila Mikhailovna
Alekseevka 2010
Introduction..…………………………………………………… …………………3
Chapter I The emergence and development of quantum physics………………………4
1.1 Quantum hypothesis……………………………………………………... 8
1.2 The theory of the atom by I. Bohr. Principle of correspondence………………………...11
Chapter II Problems of quantum mechanics…………………………………….13
1.4 The problem of interpretation of quantum mechanics.............. .16
Conclusion………………………………………………………………19
List of references…………………………………………...2 0
Introduction
According to the electromagnetic picture of the world, the world around a person is a continuous medium - a field that can have different temperatures at different points, concentrate different energy potentials, move differently, etc. A continuous medium can occupy large areas of space, its properties change continuously, and it has no sharp boundaries. These properties distinguish the field from physical bodies that have definite and clear boundaries. The division of the world into bodies and field particles, into field and space is evidence of the existence of two extreme properties of the world - discreteness and continuity. Discreteness (discontinuity) of the world means the final divisibility of the entire space-time structure into separate limited objects, properties and forms of movement, while continuity (continuity) expresses the unity, integrity and indivisibility of the object.
Within the framework of classical physics, discreteness and continuity of the world initially appear as opposite to each other, separate and independent, although in general complementary properties. In modern physics, this unity of opposites, discrete and continuous, has found its justification in the concept of wave-particle duality.
The modern quantum field picture of the world is based on a new physical theory - quantum mechanics, which describes the state and movement of micro-objects of the material world.
Chapter I. The emergence and development of quantum physics
Quantum mechanics is a theory that establishes the method of description and laws of motion of microparticles (elementary particles, atoms, molecules, atomic nuclei) and their systems, as well as the connection between quantities characterizing particles and systems with physical quantities directly measured experimentally.
The laws of quantum mechanics form the basis for the study of the structure of matter. They make it possible to clarify the structure of atoms, establish the nature of chemical bonds, explain the periodic system of elements, and study the properties of elementary particles.
Since the properties of macroscopic bodies are determined by the movement and interaction of the particles of which they are composed, the laws of quantum mechanics underlie the understanding of most macroscopic phenomena. For example, quantum mechanics made it possible to determine the structure and understand many properties of solids, to consistently explain the phenomena of ferromagnetism, superfluidity, superconductivity, to understand the nature of astrophysical objects - white dwarfs, neutron stars, and to clarify the mechanism of thermonuclear reactions in the Sun and stars.
The development of quantum mechanics dates back to the beginning of the 20th century, when physical phenomena were discovered indicating the inapplicability of Newtonian mechanics and classical electrodynamics to the processes of interaction of light with matter and processes occurring in the atom. The establishment of connections between these groups of phenomena and attempts to explain them on the basis of theory led to the discovery of the laws of quantum mechanics.
For the first time in science, ideas about quantum were expressed in 1900 by M. Planck in the process of studying the thermal radiation of bodies. Through his research, he demonstrated that energy emission occurs discretely, in certain portions - quanta, the energy of which depends on the frequency of the light wave. Planck's experiments led to the recognition of the dual nature of light, which has both corpuscular and wave properties, thus representing a dialectical unity of these opposites. Dialectics, in particular, is expressed in the fact that the shorter the wavelength of radiation, the more clearly quantum properties appear; The longer the wavelength, the brighter the wave properties of light appear.
In 1924, the French physicist L. de Broglie put forward the hypothesis that wave-particle duality is universal in nature, i.e. All particles of matter have wave properties. Later, this idea was confirmed experimentally, and the principle of wave-particle duality was extended to all processes of motion and interaction in the microworld.
In particular, N. Bohr applied the idea of energy quantization to the theory of atomic structure. According to his ideas, at the center of the atom there is a positively charged nucleus, in which almost the entire mass of the atom is concentrated, and negatively charged electrons rotate in orbits around the nucleus. Rotating electrons must lose part of their energy, which entails the unstable existence of atoms. However, in practice, atoms not only exist, but are also very stable. Explaining this issue, Bohr suggested that an electron, moving along its orbit, does not emit quanta. Radiation occurs only when an electron moves from one orbit to another, i.e. from one energy level to another, with less energy. At the moment of transition, a radiation quantum is born.
In accordance with the quantum field picture of the world, any microobject, having wave and corpuscular properties, does not have a specific trajectory of movement and cannot have certain coordinates and speed (momentum). This can only be done by determining the wave function at a given moment, and then finding its wave function at any other moment. The square of the modulus gives the probability of finding a particle at a given point in space.
In addition, the relativity of space-time in this picture of the world leads to uncertainty of coordinates and speed at a given moment, to the absence of a trajectory of movement of a micro-object. And if in classical physics the behavior of a large number of particles was subject to probabilistic laws, then in quantum mechanics the behavior of each microparticle is subject not to dynamic, but to statistical laws.
Thus, matter is two-faced: it has both corpuscular and wave properties, which manifest themselves depending on conditions. Hence, the general picture of reality in the quantum field picture of the world becomes, as it were, two-dimensional: on the one hand, it includes the characteristics of the object under study, and on the other, the observation conditions on which the certainty of these characteristics depends. This means that the picture of reality in modern physics is not only a picture of an object, but also a picture of the process of its cognition.
The idea of motion changes radically, which becomes only a special case of fundamental physical interactions. There are four types of fundamental physical interactions: gravitational, electromagnetic, strong and weak. All of them are described on the basis of the modern principle of short-range action. In accordance with it, the interaction of each type is transmitted by the corresponding field from point to point. In this case, the speed of interaction transmission is always finite and cannot exceed the speed of light in a vacuum (300,000 km/s).
The specificity of quantum field concepts of regularity and causality is that they always appear in a probabilistic form, in the form of so-called statistical laws. They correspond to a deeper level of knowledge of natural patterns. Thus, it turned out that our world is based on chance, probability.
Also, the new picture of the world for the first time included an observer, on whose presence the obtained research results depended. Moreover, the so-called anthropic principle was formulated, which states that our world is what it is only thanks to the existence of man. From now on, the emergence of man is considered a natural result of the evolution of the Universe.
THE EMERGENCE AND DEVELOPMENT OF QUANTUM PHYSICS
1.1 Quantum hypothesis
The origins of quantum physics can be found in studies of the processes of radiation of bodies. Back in 1809, P. Prevost concluded that every body radiates regardless of its environment. Development of spectroscopy in the 19th century. led to the fact that when studying emission spectra, attention is also beginning to be paid to absorption spectra. It turns out that there is a simple connection between the radiation and absorption of a body: in the absorption spectra, those parts of the spectrum that are emitted by a given body are absent or weakened. This law was explained only in quantum theory.
G. Kirchhoff in 1860 formulated a new law, which states that for radiation of the same wavelength at the same temperature, the ratio of emissivity and absorption abilities is the same for all bodies. In other words, if EλT and AλT are the emissive and absorption abilities of a body, respectively, depending on the wavelength λ and temperature T, then
where φ(λ, T) is some universal function of λ and T, the same for all bodies.
Kirchhoff introduced the concept of an absolutely black body as a body that absorbs all rays falling on it. For such a body, obviously, AλT = 1; then the universal function φ(λ, T) is equal to the emissivity of an absolutely black body. Kirchhoff himself did not determine the form of the function φ(λ, T), but only noted some of its properties.
When determining the form of the universal function φ(λ, T), it was natural to assume that one could use theoretical considerations, primarily the basic laws of thermodynamics. L. Boltzmann showed that the total radiation energy of a completely black body is proportional to the fourth power of its temperature. However, the task of specifically determining the form of the Kirchhoff function turned out to be very difficult, and research in this direction, based on thermodynamics and optics, did not lead to success.
The experiment gave a picture that cannot be explained from the point of view of classical concepts: in thermodynamic equilibrium between the oscillating atoms of matter and electromagnetic radiation, almost all the energy is concentrated in the oscillating atoms and only an insignificant part of it accounts for the radiation, whereas according to the classical theory, almost all the energy should go to the electromagnetic field.
In the 80s XIX century Empirical studies of the patterns of distribution of spectral lines and the study of the function φ(λ, T) have become more intensive and systematic. Experimental equipment has been improved. For the radiation energy of a completely black body, V. Wien in 1896 and J. Rayleigh and J. Jeans in 1900 proposed two different formulas. As experimental results have shown, the Wien formula is asymptotically correct in the region of short waves and gives sharp discrepancies with experiment in the region of long waves, and the Rayleigh-Jeans formula is asymptotically correct for long waves, but is not applicable for short waves.
In 1900, at a meeting of the Berlin Physical Society, M. Planck proposed a new formula for the distribution of energy in the spectrum of a sulfur body. This formula gave full agreement with experiment, but its physical meaning was not entirely clear. Additional analysis showed that it makes sense only if we omit that energy emission does not occur continuously, but in limited portions - quanta (ε). Moreover, ε is not any quantity, namely, ε = hν, where h is a certain constant and v is the frequency of light. This led to the recognition, along with the atomism of matter, of the atomism of energy or action, the discrete, quantum nature of radiation, which did not fit into the framework of the concepts of classical physics.
The formulation of the energy quanta hypothesis was the beginning of a new era in the development of theoretical physics. With great success, this hypothesis began to be used to explain other phenomena that could not be described on the basis of the concepts of classical physics.
An essentially new step in the development of the quantum hypothesis was the introduction of the concept of light quanta. This idea was developed in 1905 by Einstein and used by him to explain the photoelectric effect. A number of studies have provided evidence of the truth of this idea. In 1909, Einstein, continuing his research into the laws of radiation, showed that light has both wave and corpuscular properties. It became increasingly obvious that the wave-particle duality of light radiation cannot be explained from the standpoint of classical physics. In 1912, A. Poincaré finally proved the incompatibility of Planck’s formula and classical mechanics. New concepts, new ideas and a new scientific language were required so that physicists could comprehend these unusual phenomena. All this appeared later - along with the creation and development of quantum mechanics.
Chapter II Problems of quantum mechanics…………………………………….13
1.3 Creation of non-relativistic quantum mechanics………………...13
1.4 The problem of interpretation of quantum mechanics............16
Conclusion………………………………………………………………………………19
List of references……………………………………………………………...20