Basic properties of the electron. What does an electron consist of? Mass and charge of an electron. Fundamental and quantum properties of the electron
Read also
An electron is an elementary particle, which is one of the main units in the structure of matter. The electron charge is negative. The most accurate measurements were made at the beginning of the twentieth century by Millikan and Ioffe.
The electron charge is equal to minus 1.602176487 (40)*10 -1 9 C.
The electric charge of other smallest particles is measured through this value.
General concept of electron
Particle physics says that the electron is indivisible and has no structure. It is involved in electromagnetic and gravitational processes and belongs to the lepton group, just like its antiparticle, the positron. Among other leptons it has the lightest weight. If electrons and positrons collide, this results in their annihilation. Such a pair can arise from a gamma quantum of particles.
Before neutrinos were measured, the electron was considered the lightest particle. In quantum mechanics it is classified as a fermion. The electron also has a magnetic moment. If a positron is also included in it, then the positron is divided as a positively charged particle, and the electron is called a negatron, as a particle with a negative charge.

Selected properties of electrons
Electrons are classified as the first generation of leptons, with the properties of particles and waves. Each of them is endowed with a quantum state, which is determined by measuring energy, spin orientation and other parameters. His belonging to fermions is revealed through the impossibility of having two electrons in the same quantum state at the same time (according to the Pauli principle).
It is studied in the same way as a quasiparticle in a periodic crystal potential, whose effective mass can differ significantly from the mass at rest.
Through the movement of electrons, electric current, magnetism and thermal emf occur. The charge of an electron in motion forms a magnetic field. However, an external magnetic field deflects the particle from the straight direction. When accelerated, an electron acquires the ability to absorb or emit energy as a photon. Its multitude consists of electronic atomic shells, the number and position of which determine the chemical properties.
Atomic mass mainly consists of nuclear protons and neutrons, while the mass of electrons makes up about 0.06% of the total atomic weight. The electric Coulomb force is one of the main forces capable of holding an electron close to the nucleus. But when molecules are created from atoms and chemical bonds arise, electrons are redistributed in the new space formed.
Nucleons and hadrons participate in the appearance of electrons. Isotopes with radioactive properties are capable of emitting electrons. In laboratories, these particles can be studied using special instruments, and for example, telescopes can detect radiation from them in plasma clouds.

Opening
The electron was discovered by German physicists in the nineteenth century when they were studying the cathode properties of rays. Then other scientists began to study it in more detail, raising it to the rank of a separate particle. Radiation and other related physical phenomena were studied.
For example, the team led by Thomson estimated the charge of the electron and the mass of the cathode ray, the relationship of which, as they found, does not depend on the material source.
And Becquerel found that minerals emit radiation on their own, and their beta rays are able to be deflected by the action of an electric field, and the mass and charge retain the same relationship as that of cathode rays.

Atomic theory
According to this theory, an atom consists of a nucleus and electrons around it, arranged in a cloud. They are in certain quantized states of energy, the change of which is accompanied by the process of absorption or emission of photons.
Quantum mechanics
At the beginning of the twentieth century, a hypothesis was formulated according to which material particles have the properties of both particles themselves and waves. Light can also appear in the form of a wave (it is called a de Broglie wave) and particles (photons).
As a result, the famous Schrödinger equation was formulated, which described the propagation of electron waves. This approach was called quantum mechanics. It was used to calculate the electronic states of energy in the hydrogen atom.
Fundamental and quantum properties of the electron
The particle exhibits fundamental and quantum properties.
The fundamental ones include mass (9.109 * 10 -31 kilograms), elementary electric charge (that is, the minimum portion of charge). According to the measurements that have been carried out to date, the electron does not contain any elements that can reveal its substructure. But some scientists are of the opinion that it is a point-like charged particle. As indicated at the beginning of the article, the electronic electric charge is -1.602 * 10 -19 C.

While being a particle, an electron can simultaneously be a wave. An experiment with two slits confirms the possibility of its simultaneous passage through both of them. This conflicts with the properties of a particle, where passage through only one slit is possible at a time.
Electrons are considered to have the same physical properties. Therefore, their rearrangement, from the point of view of quantum mechanics, does not lead to a change in the system state. The electron wave function is antisymmetric. Therefore, its solutions vanish when identical electrons fall into the same quantum state (Pauli principle).
The specific charge of an electron (i.e., the ratio) was first measured by Thomson in 1897 using the discharge tube shown in Fig. 74.1. The electron beam coming out of the hole in anode A (cathode rays; see § 85) passed between the plates of a flat capacitor and hit the fluorescent screen, creating a luminous spot on it.
By applying voltage to the capacitor plates, it was possible to influence the beam with an almost uniform electric field. The tube was placed between the poles of an electromagnet, with the help of which it was possible to create a uniform magnetic field perpendicular to the electric one on the same section of the electron path (the area of this field is circled in Fig. 74.1 with a dotted circle). When the fields were turned off, the beam hit the screen at point O. Each of the fields separately caused the beam to shift in the vertical direction. The displacement values are determined by formulas (73.3) and (73.4) obtained in the previous paragraph.
By turning on the magnetic field and measuring the displacement of the beam trace caused by it
![]()
Thomson also turned on the electric field and selected its value so that the beam again hit point O. In this case, the electric and magnetic fields acted on the electrons of the beam simultaneously with equal but oppositely directed forces. In this case, the condition was fulfilled
Solving equations (74.1) and (74.2) together, Thomson calculated .
Bush used the magnetic focusing method to determine the specific charge of electrons. The essence of this method is as follows. Let us assume that in a uniform magnetic field, a slightly diverging beam of electrons, symmetrical relative to the direction of the field, having the same velocity v, flies out from a certain point. The directions in which electrons are emitted form small angles a with direction B. In § 72 it was found that electrons move in this case along spiral trajectories, completing in the same time
![]()
full revolution and shifting along the direction of the field at a distance equal to
![]()

Due to the smallness of the angle a, the distances (74.3) for different electrons turn out to be practically the same and equal (for small angles). Consequently, a slightly diverging beam will be focused at a point located at a distance from the electron emission point
![]()
In the Bush experiment, electrons emitted by the hot cathode K (Fig. 74.2) are accelerated through a potential difference U applied between the cathode K and anode A. As a result, they acquire a speed u, the value of which can be found from the relation
Having then flown out of the hole in the anode, the electrons form a narrow beam directed along the axis of the evacuated tube inserted inside the solenoid. A capacitor is placed at the input to the solenoid, to which an alternating voltage is applied. The field created by the capacitor deflects the electrons of the beam from the axis of the device at small angles a that change with time. This leads to “swirling” of the beam - electrons begin to move along different spiral trajectories. A fluorescent screen is placed at the outlet of the solenoid. If we select the magnetic induction B so that the distance Г from the capacitor to the screen satisfies the condition
(l is the pitch of the spiral, is an integer), then the point of intersection of the electron trajectories will hit the screen - the electron beam will be focused at this point and will excite a sharp luminous spot on the screen. If condition (74.6) is not met, the luminous spot on the screen will be blurred. Having solved equations (74.4), (74.5) and (74.6) together, we can find
The most accurate value of the specific electron charge, established taking into account the results obtained by different methods, is equal to
Value (74.7) gives the ratio of the electron charge to its rest mass. In the experiments of Thomson, Bush and other similar experiments, the ratio of charge to relativistic mass was determined equal to
![]()

In Thomson's experiments, the electron speed was approximately 0.1 s. At this speed, the relativistic mass exceeds the rest mass by 0.5%. In subsequent experiments, the electron speed reached very high values. In all cases, a decrease in the measured values with increasing v was found, which occurred in exact accordance with formula (74.8).
The charge of an electron was determined with great accuracy by Millikan in 1909. Millikan introduced tiny droplets of oil into the closed space between the horizontally located capacitor plates (Fig. 74.3). When splashed, the droplets became electrified, and they could be placed motionless by selecting the value and sign of the voltage on the capacitor.
Equilibrium occurred under the condition
here is the charge of the droplet, P is the resultant of gravity and Archimedean force, equal to
![]() (74.10)
(74.10)
( - droplet density, - its radius, - air density).
From formulas (74.9) and (74.10), knowing , it was possible to find . To determine the radius, the speed of uniform droplet fall in the absence of a field was measured. Uniform movement of the droplet is established provided that the force P is balanced by the resistance force (see formula (78.1) of the 1st volume; - air viscosity):
![]() (74.11)
(74.11)
The movement of the droplet was observed using a microscope. For the measurement, the time it took for a droplet to travel the distance between two threads visible in the field of view of the microscope was determined.
It is very difficult to accurately fix the equilibrium of a droplet. Therefore, instead of a field that met condition (74.9), a field was switched on, under the influence of which the droplet began to move upward at a low speed. The steady rate of ascent is determined from the condition that the force P and the force in total balance the force
By excluding P and from equation (74.10), (74.11) and (74.12), we obtain an expression for
![]()
(Milliken made an amendment to this formula, taking into account that the sizes of the droplets were comparable to the free path of air molecules).

So, by measuring the speed of free fall of a droplet and the speed of its rise in a known electric field, it was possible to find the charge of the droplet e. Having measured the speed at a certain value of charge, Millikan caused ionization of the air by irradiating the space between the plates with X-rays. Individual ions, adhering to the droplet, changed its charge, as a result of which the speed also changed. After measuring the new velocity value, the space between the plates was again irradiated, etc.
The changes in the charge of the droplet and the charge itself measured by Millikan each time turned out to be integer multiples of the same value. Thus, the discreteness of the electric charge was experimentally proven, i.e., the fact that every charge is composed of elementary charges of the same size.
The value of the elementary charge, established taking into account Millikan's measurements and data obtained by other methods, is equal to
PHYSICAL BASICS OF IONIC OPERATION |
|||
AND SEMICONDUCTOR DEVICES |
|||
1.1. Properties of the electron |
|||
The electric field in electronic devices accelerates or torso- |
|||
affects the movement of electrons. Let the electron e located in |
|||
electric field with intensity E, force F acts (Fig. 1.1) |
|||
F = − eE, |
|||
directed against the field force. |
|||
According to Newton's second law, the force F is equal to the product |
|||
electron mass m by acceleration a imparted to the electron by force F |
|||
in a field with intensity E: |
|||
F = ma. |
|||
From (1.1) and (1.2), the acceleration of electric |
|||
a = E e , |
|||
from equation (1.3) it is clear that c due to |
|||
change in electrical tension |
Rice. 1.1. Electron in homogeneous |
||
field changes accelerating |
electric field |
||
nieelectron. In addition, |
|||
when the field strength falls in the direction of the initial velocity v 0 |
electron |
||
moves accelerated and acquires the highest speed and kinetic |
|||
ical energy at the end of its journey. |
|||
We will find the speed v of the electron based on the known positions |
|||
physics. Firstly, the work of field forces to move electricity in it |
|||

the flow from point A to point B is the product of the charge |
|||
electron e to the potential difference of these points: |
|||
W e = (− e )(U A − U B ). |
|||
Since U B > U A, then |
|||
U A − U B = − U . |
|||
Therefore the work |
|||
We = (e)(− U ) = eU. |
|||
Secondly, according to the law of conservation of energy, the work We expended |
|||
induced by the field to move the electron is equal to the increment of the kinetic |
|||
comb energy of an electron moving in an electric field: |
|||
W = m (v 2 − v 2 ) / 2 . |
|||
Taking the initial speed v 0 = 0, from (5) we find the value |
|||
terminal electron velocity |
|||
2 W e = |
2 U e . |
||
The speed of electrons in electronic devices is significantly lower - |
|||
faster than the speed of light, therefore the ratio of quantities e /m ≈ e /m 0 |
|||
v ≈ 600 |
|||
From (1.9) it is clear that the speed of electron motion in an electrical |
|||
sk field (km/s) depends only on the potential difference between |
|||
the starting and ending points of the path traveled by the electron, and |
|||
does not depend on the shape of the path. Sometimes the speed of an electron is measured in |
|||
volts For example: the speed of the electron is 100 V. This means that the electron |
|||
the throne acquired such a speed after passing through a potential difference of 100 V. |
|||
If an electron begins its motion from a state of rest, it |
|||
will move uniformly accelerated, rectilinearly against the forces |
|||
electric field lines, absorbing energy from the field. Electric |
|||
The electric field for an electron is accelerating. |
|||
If the initial speed coincides with the direction of the force |
|||
electric field lines, such a field for an electron is a torus |
|||
moist. The speed of the electron will decrease, the energy of the electron |
|||
The rhona will also decrease (will be returned to the field). If |
|||

the size of the field allows, the electron will stop and then begin to move against the lines of force of this field.
If the initial speed is directed opposite the electric field lines, such a field for the electron is accelerating. The electric field moves positive charges in the direction of the field lines.
1.2. Types of electronic emission
The phenomenon of electron emission from the surface of a solid is called electron emission, and the source of electrons itself is called an emitter. Depending on the methods of external energy influence on electrons, causing them to leave the emitter, several types of electron emission are distinguished.
Thermionic emission occurs as a result of heating the emitter. With increasing temperature, thermal vibrations of the solid lattice occur. Due to this thermal excitation energy, part of the electrons leaves the emitter and forms an emission current. The higher the emitter temperature, the more electrons acquire such energy, as a result of which the thermionic emission current increases. The minimum temperature at which emission current appears is called critical. It depends on the emitter material.
Secondary electron emission - emission of secondary electricity
electrons from the surface of the emitter when irradiated with a flow of primary electrons. The primary electron flow incident on the secondary emitter is partially reflected from its surface and partially penetrates deep into it. Here, the primary electrons collide with the electrons of the crystal lattice of the emitter and give them part of their energy, exciting them. Some of the excited electrons escape into the external environment; these electrons are secondary.
Electrostatic electron emission (field emission)
arises from the surface of a solid or liquid body under the influence of an external accelerating electric field with high intensity (107 V/m). The greater the field strength, the greater the field emission current.
Photoelectron emission occurs when the emitter is irradiated with light. The efficiency of this type of emission depends on the wavelength (inverse relationship) and on the magnitude of the light flux (direct relationship).
An electron is a fundamental particle, one of those that are the structural units of matter. According to the classification, it is a fermion (a particle with half-integer spin, named after the physicist E. Fermi) and a lepton (particles with half-integer spin that do not participate in the strong interaction, one of the four fundamental ones in physics). The baryon is equal to zero, like other leptons.
Until recently, it was believed that the electron was an elementary, that is, indivisible, structureless particle, but now scientists have a different opinion. What does an electron consist of according to modern physicists?
History of the name
Even in Ancient Greece, naturalists noticed that amber, previously rubbed with wool, attracts small objects, that is, it exhibits electromagnetic properties. The electron got its name from the Greek ἤλεκτρον, which means “amber”. The term was proposed by J. Stoney in 1894, although the particle itself was discovered by J. Thompson in 1897. It was difficult to detect it, the reason for this is its low mass, and the charge of the electron became decisive in the discovery experiment. The first images of the particle were taken by Charles Wilson using a special camera, which is used even in modern experiments and is named after him.
An interesting fact is that one of the prerequisites for the discovery of the electron is a statement by Benjamin Franklin. In 1749, he developed a hypothesis according to which electricity is a material substance. It was in his work that such terms as positive and negative charges, capacitor, discharge, battery and particle of electricity were first used. The specific charge of an electron is considered to be negative, and that of a proton is considered positive.
Discovery of the electron
In 1846, the concept of “atom of electricity” began to be used in his works by the German physicist Wilhelm Weber. Michael Faraday discovered the term “ion”, which now, perhaps, everyone knows from their school days. The question of the nature of electricity was studied by many eminent scientists, such as the German physicist and mathematician Julius Plücker, Jean Perrin, the English physicist William Crookes, Ernst Rutherford and others.
Thus, before Joseph Thompson successfully completed his famous experiment and proved the existence of a particle smaller than an atom, many scientists worked in this field, and the discovery would not have been possible if they had not done this colossal work.

In 1906, Joseph Thompson received the Nobel Prize. The experiment consisted of the following: beams of cathode rays were passed through parallel metal plates that created an electric field. Then they had to make the same path, but through a system of coils that created a magnetic field. Thompson discovered that when exposed to an electric field, the rays were deflected, and the same was observed under magnetic influence, but the beams of cathode rays did not change their trajectories if they were acted upon by both of these fields in certain ratios, which depended on the speed of the particles.
After calculations, Thompson learned that the speed of these particles was significantly lower than the speed of light, which meant that they had mass. From that moment on, physicists began to believe that open particles of matter were part of the atom, which was later confirmed. He called it the “planetary model of the atom.”
Paradoxes of the quantum world
The question of what an electron consists of is quite complex, at least at this stage of scientific development. Before considering it, we need to address one of the paradoxes of quantum physics that even scientists themselves cannot explain. This is the famous double slit experiment that explains the dual nature of the electron.
Its essence is that in front of the “gun” that shoots particles, there is a frame with a vertical rectangular hole. Behind her there is a wall on which traces of hits will be observed. So, first you need to understand how matter behaves. The easiest way to imagine how a machine launches tennis balls. Some of the balls fall into the hole, and the marks from the hits on the wall form one vertical stripe. If you add another similar hole at some distance, the tracks will form, respectively, two stripes.
Waves behave differently in such a situation. If traces from a collision with a wave are displayed on the wall, then in the case of one hole there will also be one stripe. However, everything changes in the case of two slits. The wave passing through the holes is divided in half. If the top of one of the waves meets the bottom of the other, they cancel each other out and an interference pattern (several vertical fringes) appears on the wall. Places where waves intersect will leave a mark, but places where mutual cancellation occurred will not.

Amazing discovery
With the help of the experiment described above, scientists can clearly demonstrate to the world the difference between quantum and classical physics. When they started shooting electrons at the wall, it showed the usual vertical pattern: some particles, just like tennis balls, fell into the gap, and some did not. But everything changed when the second hole appeared. It appeared on the wall. First, physicists decided that the electrons interfered with each other, and decided to let them in one at a time. However, after a couple of hours (the speed of moving electrons is still much lower than the speed of light), the interference pattern began to appear again.
Unexpected turn
The electron, along with some other particles such as photons, exhibits wave-particle duality (the term "quantum-wave duality" is also used). Just as it is both alive and dead, the state of an electron can be both corpuscular and wave.
However, the next step in this experiment gave rise to even more mysteries: a fundamental particle, about which everything seemed to be known, presented an incredible surprise. Physicists decided to install an observation device at the holes in order to record which slit the particles pass through and how they manifest themselves as waves. But as soon as the observation mechanism was installed, only two stripes appeared on the wall, corresponding to two holes, and no interference pattern! As soon as the “surveillance” was removed, the particle again began to exhibit wave properties, as if it knew that no one was watching it anymore.
Another theory
Physicist Born suggested that a particle does not turn into a wave in the literal sense of the word. The electron “contains” a probability wave; it is this wave that gives the interference pattern. These particles have the property of superposition, that is, they can be located in any place with a certain degree of probability, which is why they can be accompanied by a similar “wave”.
Nevertheless, the result is obvious: the very fact of the presence of an observer affects the result of the experiment. It seems incredible, but this is not the only example of this kind. Physicists also conducted experiments on larger parts of matter; once the object became the thinnest piece of aluminum foil. Scientists noted that the mere fact of some measurements influenced the temperature of the object. They are not yet able to explain the nature of such phenomena.

Structure
But what does an electron consist of? At the moment, modern science cannot answer this question. Until recently, it was considered an indivisible fundamental particle, but now scientists are inclined to believe that it consists of even smaller structures.
The specific charge of an electron was also considered elementary, but quarks having a fractional charge have now been discovered. There are several theories as to what an electron is made of.
Today you can see articles that claim that scientists have succeeded in splitting an electron. However, this is only partly true.
New experiments
Back in the eighties of the last century, Soviet scientists suggested that it would be possible to split an electron into three quasiparticles. In 1996, it was possible to separate it into a spinon and a holon, and recently the physicist Van den Brink and his team separated the particle into a spinon and an orbiton. However, splitting can only be achieved under special conditions. The experiment can be carried out in extremely low temperature conditions.
When electrons “cool down” to absolute zero, which is about -275 degrees Celsius, they practically stop and form something like matter among themselves, as if merging into one particle. Under such conditions, physicists manage to observe the quasiparticles that make up the electron.

Carriers of information
The radius of the electron is very small, it is equal to 2.81794. 10 -13 cm, but it turns out that its components are much smaller in size. Each of the three parts into which the electron was “divided” carries information about it. Orbiton, as the name suggests, contains data about the orbital wave of a particle. The spinon is responsible for the spin of the electron, and the holon tells us about the charge. In this way, physicists can observe separately the different states of electrons in a highly cooled substance. They were able to trace holon-spinon and spinon-orbiton pairs, but not the whole trio together.
New technologies
The physicists who discovered the electron had to wait several decades before their discovery was put into practice. Nowadays, technologies find use within a few years; just remember graphene - an amazing material consisting of carbon atoms in one layer. How will electron splitting be useful? Scientists predict the creation of a speed that, in their opinion, is several tens of times greater than that of the most powerful modern computers.
What is the secret of quantum computer technology? This can be called simple optimization. In a conventional computer, the minimal, indivisible piece of information is a bit. And if we consider data to be something visual, then for a machine there are only two options. A bit can contain either a zero or a one, that is, parts of the binary code.
New method
Now let's imagine that a bit contains both a zero and a one - this is a “quantum bit”, or “cubit”. The role of simple variables will be played by the spin of the electron (it can rotate either clockwise or counterclockwise). Unlike a simple bit, a cubit can perform several functions simultaneously, due to this the speed of operation will increase; the low mass and charge of the electron do not matter here.
This can be explained using the example of a labyrinth. To get out of it, you need to try many different options, of which only one will be correct. A traditional computer may solve problems quickly, but still can only work on one single problem at a time. He will go through all the possible paths one by one, and eventually find a way out. A quantum computer, thanks to the duality of the cubit, can solve many problems simultaneously. He will review all possible options not in turn, but at a single point in time, and also solve the problem. The only difficulty so far is to get many quanta to work on one task - this will be the basis of a new generation computer.

Application
Most people use a computer at the everyday level. Conventional PCs are still doing an excellent job of this, but to predict events that depend on thousands, and maybe hundreds of thousands of variables, the machine must be simply huge. It can easily handle things like monthly weather forecasting, natural disaster data processing and prediction, and perform complex mathematical calculations with many variables in a fraction of a second, all with a processor the size of a few atoms. So perhaps very soon our most powerful computers will be as thin as a sheet of paper.

Staying healthy
Quantum computer technology will make a huge contribution to medicine. Humanity will have the opportunity to create nanomechanisms with the most powerful potential; with their help, it will be possible not only to diagnose diseases simply by looking at the entire body from the inside, but also to provide medical care without surgical intervention: the smallest robots with the “brains” of an excellent computer will be able to perform all operations.
A revolution in the field of computer games is inevitable. Powerful machines capable of solving problems instantly will be able to play games with incredibly realistic graphics, and fully immersive computer worlds are just around the corner.
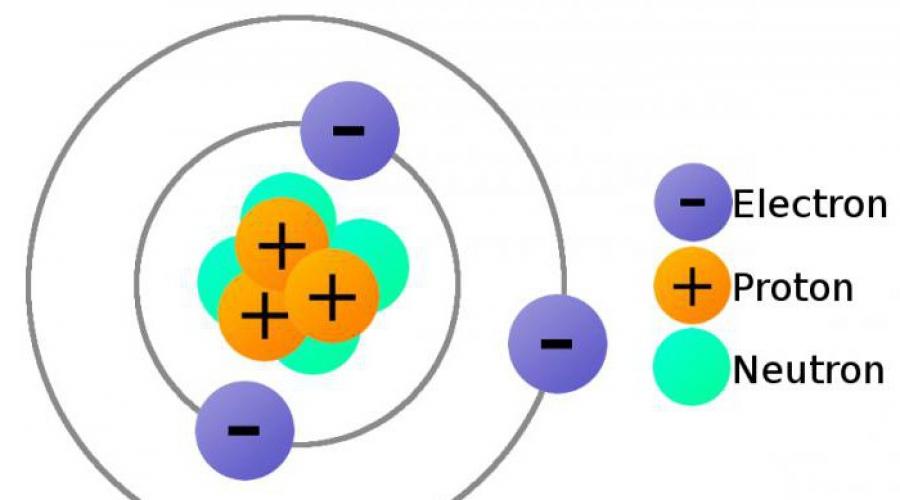
Structure of matter.
The structure of the atom.
An atom is the smallest particle of a chemical element, the bearer of all its chemical properties. An atom is chemically indivisible. Atoms can exist either in a free state or in combination with atoms of the same element or another element.
The unit of atomic and molecular masses is currently taken to be 1/12 of the mass of a carbon atom with an atomic mass of 12 (isotope). This unit is called a carbon unit.
Mass and size of atoms. Avogadro's number.
A gram atom, just like a gram molecule of any substance, contains 6.023 10^23 atoms or molecules, respectively. This number is called Avogadro's number (N0). So, in 55.85 g of iron, 63.54 g of copper, 29.98 g of aluminum, etc., there is a number of atoms equal to Avogadro’s number.
Knowing Avogadro's number, it is not difficult to calculate the mass of one atom of any element. To do this, the gram-atomic mass of one atom must be divided by 6.023 10^23. Thus, the mass of the hydrogen atom (1) and the mass of the carbon atom (2) are respectively equal: 
Based on Avogadro's number, one can estimate the volume of an atom. For example, the density of copper is 8.92 g/cm^3, and the gram-atomic mass is 63.54 g. This means that one gram-atom of copper occupies the volume  , and per one copper atom there is a volume
, and per one copper atom there is a volume  .
.
Atomic structure.
An atom is a complex formation and consists of a number of smaller particles. The atoms of all elements consist of a positively charged nucleus and electrons - negatively charged particles of very low mass. The nucleus occupies a negligible part of the total volume of the atom. The diameter of an atom is cm, and the diameter of the nucleus is cm.
Although the diameter of the nucleus of an atom is 100,000 times smaller than the diameter of the atom itself, almost the entire mass of the atom is concentrated in its nucleus. It follows that the density of atomic nuclei is very high. If it were possible to collect 1 cm3 of atomic nuclei, then its mass would be about 116 million tons.
The nucleus consists of protons and neutrons. These particles have a common name - nucleons.
Proton- - stable elementary particle with a mass close to a carbon unit. The proton charge is equal to the electrode charge, but with the opposite sign. If the charge of an electron is taken to be -1, then the charge of a proton is +1. A proton is a hydrogen atom missing an electron.
Neutron– an atomic shell, the negative charge of which compensates for the positive charge of the nucleus due to the presence of protons in it.
Thus, the number of electrons in an atom is equal to the number of protons in its nucleus.
The relationship between the number of protons, the number of neutrons and the mass number of an atom is expressed by the equation: N=A-Z
Hence, the number of neutrons in the nucleus of an atom of any element is equal to the difference between its mass number and the number of protons.
So the number of neutrons in the nucleus of a radium atom with a mass of 226 N=A-Z=226-88=138
Mass and charge of an electron.
All chemical processes of formation and destruction of chemical compounds occur without changing the nuclei of the atoms of the elements that make up these compounds. Only the electronic shells undergo changes. Chemical energy is thus related to the energy of electrons. To understand the processes of formation and destruction of chemical compounds, one should have an idea about the properties of the electron in general and especially about the properties and behavior of the electron in the atom.
Electron is an elementary particle that has an elementary negative electrical charge, i.e., the smallest amount of electricity that can exist. The charge of an electron is equal to el. Art. units or pendant. The rest mass of an electron is equal to g, i.e. 1837.14 times less than the mass of a hydrogen atom. The mass of an electron is a carbon unit.
Bohr's model of the atom.
At the beginning of the 20th century, M. Planck A. Einstein created the quantum theory of light, according to which light is a flow of individual quanta of energy carried by particles of light - photons.
Magnitude of energy quantum(E) is different for different radiations and is proportional to the oscillation frequency:
,
where h is Planck's constant.
M. Planck showed that atoms absorb or emit radiant energy only in separate, well-defined portions - quanta.
Trying to link the law of classical mechanics with quantum theory, the Danish scientist N. Bohr believed that an electron in a hydrogen atom can only be in certain - constant orbits, the radii of which are related to each other as the squares of integers ![]() These orbits were called stationary by N. Bohr.
These orbits were called stationary by N. Bohr.
Energy is emitted only when an electron moves from a more distant orbit to an orbit closer to the nucleus. When an electron moves from a close orbit to a more distant one, energy is absorbed by the atom. ![]() , where are the energies of electrons in stationary states.
, where are the energies of electrons in stationary states.
When Ei > Ek, energy is released.
When Ei< Ек энергия поглощается.
The solution to the problem of the distribution of electrons in an atom is based on the study of the line spectra of elements and their chemical properties. The spectrum of the hydrogen atom almost completely confirmed N. Bohr's theory. However, N. Bohr's theory could not explain the observed splitting of spectral lines in multielectron atoms and the intensification of this splitting in magnetic and electric fields.
Wave properties of the electron.
The laws of classical physics contrast the concepts of “particle” and “wave” with each other. Modern physical theory, called quantum, or wave mechanics, showed that the movement and interaction of particles of small mass - microparticles - occur according to laws different from the laws of classical mechanics. A microparticle simultaneously has some properties of corpuscles (particles) and some properties of waves. On the one hand, an electron, proton or other microparticle moves and acts like a corpuscle, for example, when colliding with another microparticle. On the other hand, when a microparticle moves, the phenomena of interference and diffraction typical of electromagnetic waves are revealed.
Thus, in the properties of the electron (as well as other microparticles), in the laws of its motion, the continuity and interconnection of two qualitatively different forms of existence of matter, substance and field are manifested. A microparticle cannot be considered either as an ordinary particle or as an ordinary wave. The microparticle has wave-particle duality.
Speaking about the relationship between matter and field, we can come to the conclusion that if each material particle has a certain mass, then, apparently, this same particle must also have a certain wave length. The question arises about the relationship between mass and wave. In 1924, the French physicist Louis de Broglie suggested that with every moving electron (and in general with every moving material particle) a wave process is associated, the wavelength of which is , where is the wavelength in cm (m), h is Planck’s constant, equal to ![]() erg. sec (), m - particle mass in g (kg), - particle speed, in cm/sec.
erg. sec (), m - particle mass in g (kg), - particle speed, in cm/sec.
From this equation it is clear that a particle at rest must have an infinitely long wavelength and that the wavelength decreases as the particle's speed increases. The wavelength of a moving particle of large mass is very small and cannot yet be determined experimentally. That is why we are talking about the wave properties of microparticles only. An electron has wave properties. This means that its movement in an atom can be described by a wave equation.
The planetary model of the structure of the hydrogen atom, created by N. Bohr, who proceeded from the idea of the electron only as a classical particle, cannot explain a number of properties of the electron. Quantum mechanics has shown that the idea of the movement of an electron around a nucleus in certain orbits, similar to the movement of planets around the Sun, should be considered untenable.
An electron, having the properties of a wave, moves throughout the entire volume, forming an electron cloud, which can have a different shape for electrons located in one atom. The density of this electron cloud in one or another part of the atomic volume is not the same.
Characteristics of an electron by four quantum numbers.
The main characteristic that determines the movement of an electron in the field of a nucleus is its energy. The energy of an electron, like the energy of a particle of light flux - a photon, does not take on any, but only certain discrete, discontinuous or, as they say, quantized values.
A moving electron has three degrees of freedom of movement in space (corresponding to three coordinate axes) and one additional degree of freedom, due to the presence of the electron’s own mechanical and magnetic moments, which take into account the rotation of the electron around its axis. Consequently, for a complete energy characteristic of the state of an electron in an atom, it is necessary and sufficient to have four parameters. These parameters are called quantum numbers. Quantum numbers, just like the energy of an electron, cannot reach all, but only certain values. Adjacent values of quantum numbers differ by one.
Principal quantum number n characterizes the total energy reserve of an electron or its energy level. The principal quantum number can take values of integers from 1 to . For an electron located in the field of the nucleus, the principal quantum number can take values from 1 to 7 (corresponding to the number of the period in the periodic system in which the element is located). Energy levels are designated either by numbers in accordance with the values of the principal quantum number, or by letters:
P | |||||||
Level designation |
If, for example, n=4, then the electron is on the fourth energy level, counting from the atomic nucleus, or on the N level.
Orbital quantum number l, which is sometimes called a side quantum number, characterizes the different energy states of an electron at a given level. The fine structure of the spectral lines indicates that the electrons of each energy level are grouped into sublevels. The orbital quantum number is related to the angular momentum of an electron as it moves relative to the atomic nucleus. The orbital quantum number also determines the shape of the electron cloud. The quantum number l can take all integer values from 0 to (n-1). For example, with n=4, l=0, 1, 2, 3. Each value of l corresponds to a specific sublevel. Letter designations are used for sublevels. So, when l=0, 1, 2, 3, electrons are respectively on the s-, p-, d-, f- sublevels. Electrons of different sublevels are respectively called s-, p-, d-, f - electrons. The possible number of sublevels for each energy level is equal to the number of this level, but does not exceed four. The first energy level (n=1) consists of one s-sublevel, the second (n=2), third (n=3) and fourth (n=4) energy levels respectively consist of two (s, p), three (s , p, d) and four (s, p, d, f) sublevels. There cannot be more than four sublevels, since the values l = 0, 1, 2, 3 describe the electrons of the atoms of all 104 currently known elements.
If l=0 (s-electrons), then the angular momentum of the electron relative to the atomic nucleus is zero. This can only happen when the electron moves forward not around the nucleus, but from the nucleus to the periphery and back. The electron cloud of the s-electron has the shape of a sphere.
Magnetic quantum number- The angular momentum of an electron is also associated with its magnetic moment. The magnetic quantum number characterizes the magnetic moment of an electron. The magnetic quantum number characterizes the magnetic moment of the electron and indicates the orientation of the electron cloud relative to the chosen direction or relative to the direction of the magnetic field. The magnetic quantum number can take on any positive and negative integer values, including zero, ranging from – l to + l. For example, if l=2, then it has 2 l+1=5 values (-2, -1, 0, +1, +2). When l=3 the number of values is 2 l+1=7 (-3, -2, -1, 0, +1, +2, +3). The number of values of the magnetic quantum number, which is equal to 2 l+1, is the number of energy states in which electrons of a given sublevel can exist. Thus, s-electrons have only one state (2 l+1=1), p-electrons have 3 states (2 l+1=3), d-, f-electrons have 5 and 7 states, respectively. Energy states are usually denoted schematically by energy cells, depicting them as rectangles, and electrons as arrows in these cells.
Spin quantum number- characterizes the internal motion of the electron - spin. It is associated with the electron’s own magnetic moment, caused by its motion around its axis. This quantum number can take only two values: + 1/2 and -1/2, depending on whether the magnetic field of the electron spin is oriented parallel or antiparallel to the magnetic field caused by the motion of the electron around the nucleus.
Two electrons (pairs) with the same values of quantum numbers: n, I, but with oppositely directed spins (↓) are called a paired or lone pair of electrons. Electrons with unsaturated spins () are called unpaired.
Pauli's principle, principle of least energy, Hund's rule.
The distribution of electrons in the atoms of elements is determined by three main principles: the Pauli principle, the principle of least energy and Hund's rule.
Pauli's principle. Studying numerous spectra of atoms, the Swiss physicist W. Pauli in 1925 came to a conclusion that was called the Pauli principle or prohibition: “Two electrons of an atom are prohibited from being similar to each other in all respects” or, what is the same, “in an atom there is no there may even be two electrons with the same values of all four quantum numbers." Energy states of electrons characterized by the same values of three quantum numbers: n, I and m1 are usually denoted by an energy cell.
According to the Pauli principle, an energy cell can only have two electrons, with opposite spins
The presence of a third electron in one energy cell would mean that two of them have all four quantum numbers the same. The number of possible electron states (Fig. 4) at a given sublevel is equal to the number of magnetic quantum number values for this sublevel, i.e. 21+ 1. The maximum number of electrons at this sublevel, according to the Pauli principle, will be 2(21+ 1). Thus, 2 electrons are possible in the s sublevel; the p sublevel has 6 electrons; the d sublevel has 10 electrons; there are 14 electrons in the f sublevel. The number of possible states of electrons at any level is equal to the square of the principal quantum number, and the maximum number of electrons at this level
Principle of least energy.
The sequence of placement of electrons in an atom must correspond to their greatest connection with the nucleus, i.e., the electron must have the lowest energy. Therefore, an electron does not have to occupy a higher energy level if there are places in the lower level where the electron will have less energy if located.
Since the electron energy is mainly determined by the values of the main n and orbital / quantum numbers, those sublevels for which the sum of the values of the quantum numbers n and / are smaller are filled first. For example, the energy reserve at sublevel 4s(n +/ = 4 +0 = 4) is less than at 3d(n + /= 3 + 2 = 5); 5s(n + / = 5 + 0 = 5) less than 4d(n + / = 4 + 2 = 6); 5p(n + / = 5 +1 =6) less than 4f(n + 1 = 4+3 = 7). If for two levels the sums of the values n and / are equal, then the sublevel with the smaller value n is filled first. For example, at sublevels 3d, 4p, 5s the sums of the values n and / are equal to five, in this case the sublevels with smaller values of the principal quantum number are filled first n, i.e. in the following sequence: 3d-4р-5s.
When the energies of close sublevels differ very little from each other, there are some exceptions to this rule. Thus, the 5d sublevel is filled with one electron 5dl before 4f; 6d1-2 before 5f.
Filling of energy levels and sublevels occurs in the following sequence: ls→2s→2p→3s→3p→4s→ 3d → 4p→ 5s → 4d → 5p→ 6s →(5dl) →4f→ 5d→6p→ 7s→ (6d1- 2 )→5f→6d→7p
Hund's rule.
Electrons within a given sublevel are first located, each in a separate cell, in the form of unpaired “idle” electrons. In other words, for a given value of I, the electrons in the atom are located so that their total spin number is maximum. For example, if three p-cells need to be placed three electron, then each of them will be located in a separate cell in this way:
Electronic formulas of atoms and diagrams.
Taking into account the considered provisions, it is easy to imagine the distribution of electrons across energy levels and sublevels in the atoms of any element. This distribution of electrons in an atom is written in the form of so-called electron formulas. In electronic formulas, the letters s, p, d, f denote the energy sublevels of electrons; The numbers in front of the letters indicate the energy level in which a given electron is located, and the index at the top right is the number of electrons in a given sublevel. For example, the notation 5p3 means that 3 electrons are located at the p-sublevel of the fifth energy level.
To compose the electronic formula of an atom of any element, it is enough to know the number of this element in the periodic table and follow the basic principles that govern the distribution of electrons in the atom.
Let, for example, you need to create electronic formulas for the atoms of sulfur, calcium, scandium, iron and lanthanum. From the periodic table we determine the numbers of these elements, which are respectively 16, 20, 21, 26, . This means that the energy levels and sublevels of the atoms of these elements contain 16, 20, 21, 26, 57 electrons, respectively. Observing the Pauli principle and the principle of least energy, i.e. the sequence of filling energy levels and sublevels, it is possible to compose electronic formulas for the atoms of these elements:

The structure of the electron shell of an atom can also be depicted in the form of a diagram of the arrangement of electrons in energy cells.
For iron atoms, this scheme has the following form:

This diagram clearly shows the implementation of Hund's rule. At the 3d sublevel, the maximum number of cells (four) is filled with unpaired electrons. The image of the structure of the electron shell in an atom in the form of electronic formulas and in the form of diagrams does not clearly reflect the wave properties of the electron. However, it should be remembered that each s-, p-, d-, f-electron has its own electron cloud. A different shape of an electron cloud indicates that an electron has a different probability of being in a given region of atomic space. Depending on the value of the magnetic quantum number m1, the orientation of the electron cloud in space will also be different.