Mn in the periodic table. Periodic Table of Chemical Elements
The nineteenth century in the history of mankind is a century in which many sciences were reformed, including chemistry. It was at this time that Mendeleev's periodic system appeared, and with it the periodic law. It was he who became the basis modern chemistry. The periodic system of D.I. Mendeleev is a systematization of elements that establishes the dependence of chemical and physical properties on the structure and charge of the atom of a substance.
Story
The beginning of the periodic period was laid by the book “The Correlation of Properties with the Atomic Weight of Elements,” written in the third quarter of the 17th century. It displayed the basic concepts of relatively well-known chemical elements(at that time there were only 63 of them). Moreover, many of them atomic masses were defined incorrectly. This greatly interfered with the discovery of D.I. Mendeleev.
Dmitry Ivanovich began his work by comparing the properties of elements. First of all, he worked on chlorine and potassium, and only then moved on to working with alkali metals. Armed with special cards on which chemical elements were depicted, he repeatedly tried to assemble this “mosaic”: laying it out on his table in search of the necessary combinations and matches.
After much effort, Dmitry Ivanovich finally found the pattern he was looking for and arranged the elements in periodic rows. Having received as a result empty cells between the elements, the scientist realized that not all chemical elements were known to Russian researchers, and that it was he who must give this world the knowledge in the field of chemistry that had not yet been given by his predecessors.

Everyone knows the myth that the periodic table appeared to Mendeleev in a dream, and he collected the elements from memory. unified system. This is, roughly speaking, a lie. The fact is that Dmitry Ivanovich worked quite long and concentrated on his work, and it exhausted him greatly. While working on the system of elements, Mendeleev once fell asleep. When he woke up, he realized that he had not finished the table and rather continued filling in the empty cells. His acquaintance, a certain Inostrantsev, a university teacher, decided that the periodic table had been dreamed of by Mendeleev and spread this rumor among his students. This is how this hypothesis emerged.
Fame
Mendeleev's chemical elements are a reflection of the periodic law created by Dmitry Ivanovich back in the third quarter of the 19th century (1869). It was in 1869 that Mendeleev’s notification about the creation of a certain structure was read out at a meeting of the Russian chemical community. And in the same year, the book “Fundamentals of Chemistry” was published, in which Mendeleev’s periodic system of chemical elements was published for the first time. And in the book " Natural system elements and its use to indicate the qualities of undiscovered elements” D.I. Mendeleev first mentioned the concept of “periodic law”.

Structure and rules for placing elements
The first steps in creating the periodic law were taken by Dmitry Ivanovich back in 1869-1871, at that time he worked hard to establish the dependence of the properties of these elements on the mass of their atom. Modern version represents elements summarized in a two-dimensional table.
The position of an element in the table carries a certain chemical and physical meaning. By the location of an element in the table, you can find out what its valency is and determine other chemical features. Dmitry Ivanovich tried to establish a connection between elements, both similar in properties and differing.

He based the classification of chemical elements known at that time on valence and atomic mass. By comparing the relative properties of elements, Mendeleev tried to find a pattern that would unite all known chemical elements into one system. By arranging them based on increasing atomic masses, he still achieved periodicity in each of the rows.
Further development of the system
The periodic table, which appeared in 1969, has been refined more than once. With the advent of noble gases in the 1930s, it was possible to reveal a new dependence of elements - not on mass, but on atomic number. Later, it was possible to establish the number of protons in atomic nuclei, and it turned out that it coincides with the atomic number of the element. Scientists of the 20th century studied electronic energy. It turned out that it also affects periodicity. This greatly changed ideas about the properties of elements. This point was reflected in later editions of Mendeleev’s periodic table. Each new discovery of the properties and characteristics of elements fit organically into the table.
Characteristics of Mendeleev's periodic system
The periodic table is divided into periods (7 rows arranged horizontally), which, in turn, are divided into large and small. The period begins with an alkali metal and ends with an element with non-metallic properties.
Dmitry Ivanovich's table is vertically divided into groups (8 columns). Each of them in the periodic table consists of two subgroups, namely the main and secondary ones. After much debate, at the suggestion of D.I. Mendeleev and his colleague U. Ramsay, it was decided to introduce the so-called zero group. It includes inert gases (neon, helium, argon, radon, xenon, krypton). In 1911, scientists F. Soddy were asked to place indistinguishable elements, the so-called isotopes, in the periodic table - separate cells were allocated for them.

Despite the correctness and accuracy of the periodic system, the scientific community did not want to recognize this discovery for a long time. Many great scientists ridiculed the work of D.I. Mendeleev and believed that it was impossible to predict the properties of an element that had not yet been discovered. But after the supposed chemical elements were discovered (and these were, for example, scandium, gallium and germanium), the Mendeleev system and his periodic law became the science of chemistry.
Table in modern times
Mendeleev's periodic table of elements is the basis of most chemical and physical discoveries related to atomic-molecular science. Modern concept element was formed precisely thanks to the great scientist. The emergence of Mendeleev's periodic system introduced fundamental changes in ideas about various connections And simple substances Oh. The creation of the periodic table by scientists had a huge impact on the development of chemistry and all sciences related to it.
You've probably all seen the periodic table of elements. It is possible that she still haunts you in your dreams, or maybe for now she is just a visual background decorating the wall school class. However, there is much more to this seemingly random collection of cells than meets the eye.
The periodic table (or PT, as we'll call it from time to time throughout this article), and the elements that make up it, have features that you may never have guessed. From creating the table to adding the final elements to it, here are ten facts that most people don't know.
10. Mendeleev received help
The periodic table has been in use since 1869, when it was compiled by the heavily bearded Dimitri Mendeleev. Most people think that Mendeleev was the only one who worked on this table, and thanks to this he became the most brilliant chemist of the century. However, his efforts were aided by several European scientists who made important contributions to the completion of this colossal set of elements.
Mendeleev is widely known as the father periodic table, but when he compiled it, not all elements of the table were already open. How did this become possible? Scientists are famous for their madness...
9. Latest added items

Believe it or not, the periodic table hasn't changed much since the 1950s. However, on December 2, 2016, four new elements were added at once: nihonium (element No. 113), moscovium (element No. 115), tennessine (element No. 117) and oganesson (element No. 118). These new elements only received their names in June 2016, as a five-month review was required before they were officially added to the PT.
Three elements were named after the cities or states in which they were obtained, and Oganesson was named after Russian nuclear physicist Yuri Oganesyan for his contribution to obtaining this element.
8. Which letter is not in the table?

There are 26 letters in the Latin alphabet, and each of them is important. However, Mendeleev decided not to notice this. Take a look at the table and tell me which letter is unlucky? Hint: search in order and bend your fingers after each letter you find. As a result, you will find the “missing” letter (if you have all ten fingers on your hands). Did you guess it? This is letter number 10, the letter "J".
They say that “one” is the number of lonely people. So, maybe we should call the letter “J” the letter of singles? But here's a fun fact: the majority of boys born in the United States in 2000 were given names starting with this letter. Thus, this letter did not remain without due attention.
7. Synthesized elements

As you may already know, there are currently 118 elements in the periodic table. Can you guess how many of these 118 elements were obtained in the laboratory? Of everything general list V natural conditions only 90 elements can be found.
Do you think that 28 artificially created elements is a lot? Well, just take my word for it. They have been synthesized since 1937, and scientists continue to do so today. You can find all these elements in the table. Look at elements 95 to 118, all of these elements are not found on our planet and were synthesized in laboratories. The same applies to elements numbered 43, 61, 85 and 87.
6. 137th element

In the mid-20th century, a famous scientist named Richard Feynman made a rather loud statement that shocked everyone. scientific world of our planet. According to him, if we ever discover element 137, we will not be able to determine the number of protons and neutrons in it. The number 1/137 is notable because it is the value of the fine structure constant, which describes the probability of an electron absorbing or emitting a photon. Theoretically, element #137 should have 137 electrons and a 100 percent chance of absorbing a photon. Its electrons will rotate at the speed of light. Even more incredible, element 139's electrons must spin faster than the speed of light to exist.
Are you tired of physics yet? You may be interested to know that the number 137 brings together three important areas of physics: the theory of the speed of light, quantum mechanics and electromagnetism. Since the early 1900s, physicists have speculated that the number 137 may be the basis of the Great unified theory, which will include all three of the above areas. Admittedly, this sounds as incredible as the legends of UFOs and the Bermuda Triangle.
5. What can you say about the names?

Almost all the names of the elements have some meaning, although it is not immediately clear. The names of new elements are not given arbitrarily. I would just name the element with the first word that came to my mind. For example, "kerflump". Not bad in my opinion.
Typically, element names fall into one of five main categories. The first is the names of famous scientists, classic version- einsteinium. In addition, elements may get their names depending on the places where they were first recorded, for example, germanium, americium, gallium, etc. As additional option the names of the planets are used. The element uranium was first discovered shortly after the planet Uranus was discovered. Elements can have names associated with mythology, for example there is titanium, named after the ancient Greek titans, and thorium, named after the Norse god of thunder (or star "avenger", depending on what you prefer).
And finally, there are names that describe the properties of the elements. Argon comes from the Greek word "argos", which means "lazy" or "slow". The name suggests that this gas is not active. Bromine is another element whose name comes from a Greek word. "Bromos" means "stench," and it pretty much describes the smell of bromine.
4. Was creating the table an “eureka moment”?

if you love card games, then this fact is for you. Mendeleev needed to somehow order all the elements and find a system for this. Naturally, to create a table of categories, he turned to solitaire (well, what else?) Mendeleev wrote down the atomic weight of each element on a separate card, and then began to lay out his advanced solitaire game. He arranged the elements according to their specific properties and then arranged them in each column according to their atomic weight.
Many people cannot play regular solitaire, so this solitaire game is impressive. What will happen next? Probably someone, with the help of chess, will revolutionize astrophysics or create a rocket capable of reaching the outskirts of the galaxy. It seems that there will be nothing unusual in this, considering that Mendeleev was able to obtain such an ingenious result with just a deck of ordinary playing cards.
3. Unlucky noble gases

Remember how we classified argon as the laziest and slowest element in the history of our universe? It seems that Mendeleev was overcome by the same feelings. When pure argon was first obtained in 1894, it did not fit into any of the columns of the table, so instead of searching for a solution, the scientist decided to simply deny its existence.
Even more strikingly, argon was not the only element that initially suffered this fate. In addition to argon, five other elements remained unclassified. This affected radon, neon, krypton, helium and xenon - and everyone denied their existence simply because Mendeleev could not find a place for them in the table. After several years of regrouping and reclassification, these elements (called inert gases) was still lucky enough to join a worthy club recognized as really existing.
2. Atomic love

Advice for all those who consider themselves romantics. Take a paper copy of the periodic table and cut out all the complicated and relatively unnecessary middle columns so that you are left with 8 columns (you will have a "short" form of the table). Fold it in the middle of group IV - and you will find out which elements can form compounds with each other.
Elements that “kiss” when folded are able to form stable compounds. These elements have complementary electronic structures and will combine with each other. And if this is not true love, like Romeo and Juliet or Shrek and Fiona, then I don’t know what love is.
1. Carbon rules

Carbon is trying to be at the center of the game. You think you know everything about carbon, but you don't; it's much more important than you realize. Did you know that it is present in more than half of all known compounds? And what about the fact that 20 percent of the weight of all living organisms is carbon? It's really weird, but brace yourself: every carbon atom in your body was once part of a faction carbon dioxide in the atmosphere. Carbon is not only the superelement of our planet, it is the fourth most abundant element in the entire Universe.
If the periodic table is like a party, then carbon is the main host. And it seems that he is the only one who knows how to organize everything correctly. Well, among other things, this is the main element of all diamonds, so for all its intrusiveness, it also sparkles!
The discovery of the periodic table of chemical elements by Dmitri Mendeleev in March 1869 was a real breakthrough in chemistry. The Russian scientist managed to systematize knowledge about chemical elements and present them in the form of a table, which schoolchildren are still required to study in chemistry lessons. The periodic table became the foundation for the rapid development of this complex and interesting science, and the history of its discovery is shrouded in legends and myths. For all those interested in science, it will be interesting to know the truth about how Mendeleev discovered the table of periodic elements.
History of the periodic table: how it all began
Attempts to classify and systematize known chemical elements were made long before Dmitry Mendeleev. Such famous scientists as Döbereiner, Newlands, Meyer and others proposed their systems of elements. However, due to a lack of data on chemical elements and their correct atomic masses, the proposed systems were not entirely reliable.
The history of the discovery of the periodic table begins in 1869, when a Russian scientist at a meeting of the Russian Chemical Society told his colleagues about his discovery. In the table proposed by the scientist, chemical elements were arranged depending on their properties, provided by the size of their molecular weight.
An interesting feature of the periodic table was also the presence of empty cells, which in the future were filled with open chemical elements predicted by the scientist (germanium, gallium, scandium). Since the discovery of the periodic table, additions and amendments have been made to it many times. Together with the Scottish chemist William Ramsay, Mendeleev added a group of inert gases (group zero) to the table.
Subsequently, the history of Mendeleev's periodic table was directly related to discoveries in another science - physics. Work on the table of periodic elements continues to this day, and modern scientists add new chemical elements as they are discovered. The importance of Dmitry Mendeleev’s periodic system is difficult to overestimate, since thanks to it:
- Knowledge about the properties of already discovered chemical elements was systematized;
- It became possible to predict the discovery of new chemical elements;
- Such branches of physics as atomic physics and nuclear physics began to develop;
There are many options for depicting chemical elements according to the periodic law, but the most famous and common option is the periodic table familiar to everyone.
Myths and facts about the creation of the periodic table
The most common misconception in the history of the discovery of the periodic table is that the scientist saw it in a dream. In fact, Dmitri Mendeleev himself refuted this myth and stated that he had been pondering the periodic law for many years. To systematize the chemical elements, he wrote out each of them on a separate card and repeatedly combined them with each other, arranging them in rows depending on their similar properties.
The myth about the scientist’s “prophetic” dream can be explained by the fact that Mendeleev worked on the systematization of chemical elements for days on end, interrupted by short sleep. However, only the hard work and natural talent of the scientist gave the long-awaited result and provided Dmitry Mendeleev with worldwide fame.
Many students at school, and sometimes at university, are forced to memorize or at least roughly navigate the periodic table. To do this, a person must not only have good memory, but also to think logically, linking elements into separate groups and classes. Studying the table is easiest for those people who constantly keep their brain in good shape by undergoing training on BrainApps.
Anyone who went to school remembers that one of the compulsory subjects to study was chemistry. You might like her, or you might not like her - it doesn't matter. And it is likely that much knowledge in this discipline has already been forgotten and is not used in life. However, everyone probably remembers D.I. Mendeleev’s table of chemical elements. For many, it has remained a multi-colored table, where certain letters are written in each square, indicating the names of chemical elements. But here we will not talk about chemistry as such, and describe hundreds chemical reactions and processes, but we’ll tell you how the periodic table appeared in the first place - this story will be interesting to any person, and indeed to all those who are hungry for interesting and useful information.
A little background
Back in 1668, the outstanding Irish chemist, physicist and theologian Robert Boyle published a book in which many myths about alchemy were debunked, and in which he discussed the need to search for indecomposable chemical elements. The scientist also gave a list of them, consisting of only 15 elements, but admitted the idea that there may be more elements. This became the starting point not only in the search for new elements, but also in their systematization.
A hundred years later, the French chemist Antoine Lavoisier compiled a new list, which already included 35 elements. 23 of them were later found to be indecomposable. But the search for new elements continued by scientists around the world. AND main role The famous Russian chemist Dmitry Ivanovich Mendeleev played a role in this process - he was the first to put forward the hypothesis that there could be a relationship between the atomic mass of elements and their location in the system.
Thanks to painstaking work and comparison of chemical elements, Mendeleev was able to discover the connection between the elements, in which they can be one, and their properties are not something taken for granted, but represent a periodically repeating phenomenon. As a result, in February 1869, Mendeleev formulated the first periodic law, and already in March his report “Relationship of properties with the atomic weight of elements” was presented to the Russian Chemical Society by the historian of chemistry N. A. Menshutkin. Then, in the same year, Mendeleev’s publication was published in the journal “Zeitschrift fur Chemie” in Germany, and in 1871, another German journal “Annalen der Chemie” published a new extensive publication by the scientist dedicated to his discovery.
Creating the periodic table

By 1869, the main idea had already been formed by Mendeleev, and in a fairly short time, but for a long time he could not formalize it into any orderly system that would clearly display what was what. In one of the conversations with his colleague A.A. Inostrantsev, he even said that he had everything already worked out in his head, but he couldn’t put everything into a table. After this, according to Mendeleev’s biographers, he began painstaking work on his table, which lasted three days without breaks for sleep. They tried all sorts of ways to organize elements into a table, and the work was also complicated by the fact that at that time science did not yet know about all the chemical elements. But, despite this, the table was still created, and the elements were systematized.
The legend of Mendeleev's dream
Many have heard the story that D.I. Mendeleev dreamed about his table. This version was actively disseminated by the aforementioned Mendeleev’s associate A. A. Inostrantsev as funny story with which he entertained his students. He said that Dmitry Ivanovich went to bed and in a dream clearly saw his table, in which all the chemical elements were arranged in in the right order. After this, the students even joked that 40° vodka was discovered in the same way. But there were still real prerequisites for the story with sleep: as already mentioned, Mendeleev worked on the table without sleep or rest, and Inostrantsev once found him tired and exhausted. During the day, Mendeleev decided to take a short rest, and some time later, he woke up abruptly, immediately took a piece of paper and drew a ready-made table on it. But the scientist himself refuted this whole story with the dream, saying: “I’ve been thinking about it, maybe for twenty years, and you think: I was sitting and suddenly... it’s ready.” So the legend of the dream may be very attractive, but the creation of the table was only possible through hard work.

Further work
Between 1869 and 1871, Mendeleev developed the ideas of periodicity toward which the scientific community was inclined. And one of important stages this process there was an understanding that any element in the system should have, based on the totality of its properties in comparison with the properties of other elements. Based on this, and also relying on the results of research into changes in glass-forming oxides, the chemist was able to make corrections to the values of the atomic masses of some elements, including uranium, indium, beryllium and others.
Mendeleev, of course, wanted to quickly fill the empty cells that remained in the table, and in 1870 he predicted that chemical elements unknown to science would soon be discovered, the atomic masses and properties of which he was able to calculate. The first of these were gallium (discovered in 1875), scandium (discovered in 1879) and germanium (discovered in 1885). Then the forecasts continued to be realized, and eight more new elements were discovered, including: polonium (1898), rhenium (1925), technetium (1937), francium (1939) and astatine (1942-1943). By the way, in 1900, D.I. Mendeleev and the Scottish chemist William Ramsay came to the conclusion that the table should also include elements of group zero - until 1962 they were called inert gases, and after that - noble gases.
Organization of the periodic table
Chemical elements in D.I. Mendeleev’s table are arranged in rows, in accordance with the increase in their mass, and the length of the rows is selected so that the elements in them have similar properties. For example, noble gases such as radon, xenon, krypton, argon, neon and helium are difficult to react with other elements and also have low chemical reactivity, which is why they are located in the far right column. And the elements in the left column (potassium, sodium, lithium, etc.) react well with other elements, and the reactions themselves are explosive. Simply put, within each column, elements have similar properties that vary from one column to the next. All elements up to No. 92 are found in nature, and from No. 93 they begin artificial elements, which can only be created in laboratory conditions.
In its original version, the periodic system was understood only as a reflection of the order existing in nature, and there were no explanations as to why everything should be this way. And only when she appeared quantum mechanics, the true meaning of the order of elements in the table became clear.
Lessons in the creative process
Talking about what lessons creative process can be extracted from the entire history of the creation of the periodic table by D.I. Mendeleev, we can cite as an example the ideas of an English researcher in the field creative thinking Graham Wallace and the French scientist Henri Poincaré. Let's give them briefly.
According to the studies of Poincaré (1908) and Graham Wallace (1926), there are four main stages of creative thinking:
- Preparation– the stage of formulating the main problem and the first attempts to solve it;
- Incubation– a stage during which there is a temporary distraction from the process, but work on finding a solution to the problem is carried out on a subconscious level;
- Insight– the stage at which the intuitive solution is located. Moreover, this solution can be found in a situation that is completely unrelated to the problem;
- Examination– the stage of testing and implementation of a solution, at which this solution is tested and its possible further development.
As we can see, in the process of creating his table, Mendeleev intuitively followed precisely these four stages. How effective this is can be judged by the results, i.e. by the fact that the table was created. And given that its creation was a huge step forward not only for chemical science, but also for all of humanity, the above four stages can be applied to both the implementation small projects, and to the implementation of global plans. The main thing to remember is that not a single discovery, not a single solution to a problem can be found on its own, no matter how much we want to see them in a dream and no matter how much we sleep. In order for something to work out, it doesn’t matter whether it’s creating a table of chemical elements or developing a new marketing plan, you need to have certain knowledge and skills, as well as skillfully use your potential and work hard.
We wish you success in your endeavors and successful implementation of your plans!
Even at school, sitting in chemistry lessons, we all remember the table on the wall of the classroom or chemical laboratory. This table contained a classification of all chemical elements known to mankind, those fundamental components that make up the Earth and the entire Universe. Then we could not even think that Mendeleev table is undoubtedly one of the greatest scientific discoveries, which is the foundation of our modern knowledge about chemistry.
Periodic table of chemical elements by D. I. Mendeleev
At first glance, her idea looks deceptively simple: organize chemical elements in order of increasing weight of their atoms. Moreover, in most cases it turns out that chemical and physical properties each element is similar to its previous element in the table. This pattern appears for all elements except the very first few, simply because they do not have in front of them elements similar to them in atomic weight. It is thanks to the discovery of this property that we can place a linear sequence of elements in a table much like a wall calendar, and thus combine a huge number of types of chemical elements in a clear and coherent form. Of course, today we use the concept of atomic number (the number of protons) in order to order the system of elements. This helped solve the so-called technical problem“a pair of permutations,” however, did not lead to a fundamental change in the appearance of the periodic table.
IN periodic table all elements are ordered based on their atomic number, electronic configuration, and repeating chemical properties. The rows in the table are called periods, and the columns are called groups. The first table, dating back to 1869, contained only 60 elements, but now the table had to be enlarged to accommodate the 118 elements we know today.
Mendeleev's periodic table systematizes not only the elements, but also their most diverse properties. It is often enough for a chemist to have the Periodic Table in front of his eyes in order to correctly answer many questions (not only exam questions, but also scientific ones).
The YouTube ID of 1M7iKKVnPJE is invalid.
Periodic law
There are two formulations periodic law chemical elements: classical and modern.
Classical, as presented by its discoverer D.I. Mendeleev: the properties of simple bodies, as well as the forms and properties of compounds of elements, are periodically dependent on the values of the atomic weights of the elements.
Modern: the properties of simple substances, as well as the properties and forms of compounds of elements, are periodically dependent on the charge of the nucleus of the atoms of the elements (ordinal number).
A graphic representation of the periodic law is the periodic system of elements, which represents natural classification chemical elements, based on regular changes in the properties of elements depending on the charges of their atoms. The most common images of the periodic table of elements are D.I. Mendeleev's forms are short and long.

Groups and periods of the Periodic Table
In groups are called vertical rows in the periodic table. In groups, elements are combined by attribute highest degree oxidation in oxides. Each group consists of a main and secondary subgroup. The main subgroups include elements of small periods and elements of large periods with the same properties. Side subgroups consist only of elements of large periods. The chemical properties of the elements of the main and secondary subgroups differ significantly.
Period called a horizontal row of elements arranged in order of increasing atomic numbers. There are seven periods in the periodic system: the first, second and third periods are called small, they contain 2, 8 and 8 elements, respectively; the remaining periods are called large: in the fourth and fifth periods there are 18 elements, in the sixth - 32, and in the seventh (not yet completed) - 31 elements. Each period, except the first, begins with an alkali metal and ends with a noble gas.
Physical meaning of the serial number chemical element: the number of protons in the atomic nucleus and the number of electrons orbiting around atomic nucleus, are equal to the ordinal number of the element.
Properties of the periodic table
Let us remind you that groups are called vertical rows in the periodic table and Chemical properties elements of the main and secondary subgroups differ significantly.
The properties of elements in subgroups naturally change from top to bottom:
- are intensifying metallic properties and non-metallic ones weaken;
- the atomic radius increases;
- the strength of bases and oxygen-free acids formed by the element increases;
- electronegativity decreases.
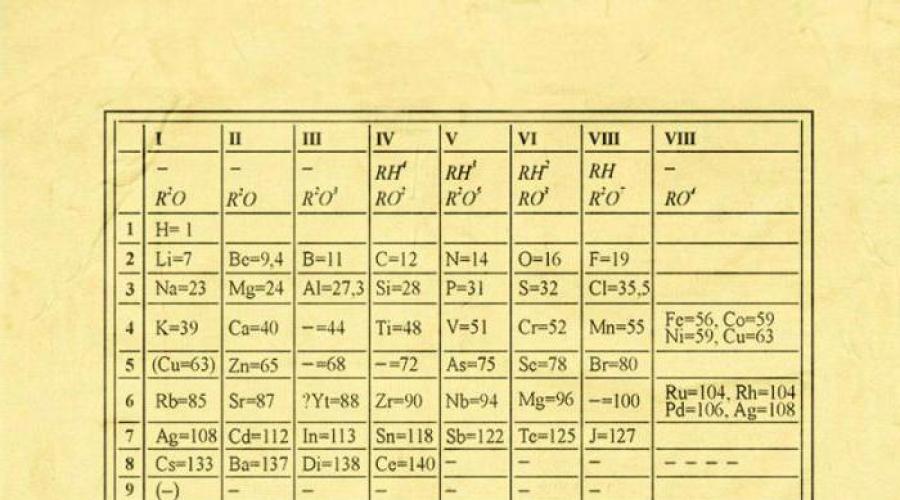
All elements except helium, neon and argon form oxygen compounds; there are only eight forms of oxygen compounds. In the periodic table they are often depicted general formulas, located under each group in increasing order of the oxidation state of the elements: R 2 O, RO, R 2 O 3, RO 2, R 2 O 5, RO 3, R 2 O 7, RO 4, where the symbol R denotes an element of this group. The formulas of higher oxides apply to all elements of the group, except in exceptional cases when the elements do not exhibit an oxidation state equal to the group number (for example, fluorine).
Oxides of the composition R 2 O exhibit strong basic properties, and their basicity increases with increasing atomic number; oxides of the composition RO (with the exception of BeO) exhibit basic properties. Oxides of the composition RO 2, R 2 O 5, RO 3, R 2 O 7 exhibit acidic properties, and their acidity increases with increasing atomic number.
The elements of the main subgroups, starting from group IV, form gaseous hydrogen compounds. There are four forms of such compounds. They are located under the elements of the main subgroups and are represented by general formulas in the sequence RH 4, RH 3, RH 2, RH.
RH 4 compounds are neutral in nature; RH 3 - weakly basic; RH 2 - slightly acidic; RH - strongly acidic character.
Let us remind you that period called a horizontal row of elements arranged in order of increasing atomic numbers.
Within a period with increasing element serial number:
- electronegativity increases;
- metallic properties decrease, non-metallic properties increase;
- the atomic radius decreases.

Elements of the periodic table
Alkali and alkaline earth elements
These include elements from the first and second groups of the periodic table. Alkali metals from the first group - soft metals, silver in color, easy to cut with a knife. They all have a single electron in their outer shell and react perfectly. Alkaline earth metals from the second group also have a silvery tint. Two electrons are placed at the outer level, and, accordingly, these metals interact less readily with other elements. Compared to alkali metals, alkaline earth metals melt and boil at higher temperatures.
Show/Hide text
Lanthanides (rare earth elements) and actinides
Lanthanides- a group of elements originally found in rare minerals; hence their name "rare earth" elements. Subsequently, it turned out that these elements are not as rare as initially thought, and therefore the name lanthanides was given to rare earth elements. Lanthanides and actinides occupy two blocks, which are located under the main table of elements. Both groups include metals; all lanthanides (except promethium) are non-radioactive; actinides, on the contrary, are radioactive.
Show/Hide text
Halogens and noble gases
The halogens and noble gases are grouped into groups 17 and 18 of the periodic table. Halogens are non-metallic elements, they all have seven electrons in their outer shell. IN noble gases All the electrons are in the outer shell, so they hardly participate in the formation of compounds. These gases are called “noble” gases because they rarely react with other elements; that is, they refer to members of a noble caste who have traditionally shunned other people in society.
Show/Hide text
Transition metals
Transition metals occupy groups 3-12 in the periodic table. Most of them are dense, hard, with good electrical and thermal conductivity. Their valence electrons (with the help of which they are connected to other elements) are located in several electron shells.
Show/Hide text
| Transition metals |
| Scandium Sc 21 |
| Titan Ti 22 |
| Vanadium V 23 |
| Chrome Cr 24 |
| Manganese Mn 25 |
| Iron Fe 26 |
| Cobalt Co 27 |
| Nickel Ni 28 |
| Copper Cu 29 |
| Zinc Zn 30 |
| Yttrium Y 39 |
| Zirconium Zr 40 |
| Niobium Nb 41 |
| Molybdenum Mo 42 |
| Technetium Tc 43 |
| Ruthenium Ru 44 |
| Rhodium Rh 45 |
| Palladium Pd 46 |
| Silver Ag 47 |
| Cadmium Cd 48 |
| Lutetium Lu 71 |
| Hafnium Hf 72 |
| Tantalum Ta 73 |
| Tungsten W 74 |
| Rhenium Re 75 |
| Osmium Os 76 |
| Iridium Ir 77 |
| Platinum Pt 78 |
| Gold Au 79 |
| Mercury Hg 80 |
| Lawrence Lr 103 |
| Rutherfordium Rf 104 |
| Dubnium Db 105 |
| Seaborgium Sg 106 |
| Borium Bh 107 |
| Hassiy Hs 108 |
| Meitnerium Mt 109 |
| Darmstadt Ds 110 |
| X-ray Rg 111 |
| Copernicium Cn 112 |
Metalloids
Metalloids occupy groups 13-16 of the periodic table. Metalloids such as boron, germanium and silicon are semiconductors and are used to make computer chips and fees.
Show/Hide text
Post-transition metals
Elements called post-transition metals, belong to groups 13-15 of the periodic table. Unlike metals, they do not have shine, but have a matte color. Compared to transition metals, post-transition metals are softer and have more low temperature melting and boiling, higher electronegativity. Their valence electrons, with which they attach other elements, are located only on the outer electron shell. Elements of the post-transition metal group have much more high temperature boiling point than metalloids.
Now consolidate your knowledge by watching a video about the periodic table and more.
Great, the first step on the path to knowledge has been taken. Now you are more or less oriented in the periodic table and this will be very useful to you, because the Periodic System of Mendeleev is the foundation on which this amazing science stands.