Who proposed the planetary model of the atom. Who proposed the nuclear model of the structure of the atom? Nuclear model of atomic structure and its diagram
One of the first models of atomic structure was proposed J. Thomson in 1904, the Atom was imagined as a “sea of positive electricity” with electrons oscillating in it. The total negative charge of the electrons of an electrically neutral atom was equal to its total positive charge.
Rutherford's experience
To test Thomson's hypothesis and more precise definition atomic structure E. Rutherford organized a series of experiments on scattering α -particles with thin metal plates - foil. In 1910, Rutherford students Hans Geiger And Ernest Marsden α conducted bombing experiments - fine particles metal plates α . They found that most
-particles pass through the foil without changing their trajectory. And this was not surprising if we accept the correctness of Thomson's model of the atom. α Source α - radiation was placed in a lead cube with a channel drilled in it, so that it was possible to obtain a flux -particles flying in a certain direction. Alpha particles are doubly ionized helium atoms ( Not 2+ α ). They have a +2 positive charge and a mass almost 7350 times the mass of an electron. Getting on the screen coated with zinc sulfide, α -particles caused it to glow, and with a magnifying glass one could see and count the individual flashes that appeared on the screen when each one hit it α -particles. Foil was placed between the radiation source and the screen. From the flashes on the screen one could judge the scattering
-particles, i.e. about their deviation from the original direction when passing through a layer of metal. α It turned out that the majority α -particles pass through the foil without changing their direction, although the thickness of the foil corresponded to hundreds of thousands of atomic diameters. But some -particles still deviated by small angles α , and occasionally α -particles abruptly changed the direction of their movement and even (about 1 in 100,000) were thrown back, as if they had encountered a massive obstacle. Cases of such a sharp deviation
From the results of this experiment the following conclusions could be drawn:
- There is some "obstacle" in the atom, which was called the nucleus.
- The nucleus has a positive charge (otherwise positively charged α -particles would not be reflected back).
- The nucleus has very small dimensions compared to the size of the atom itself (only a small part α -particles changed direction of movement).
- The nucleus has a large mass compared to the mass α -particles
Rutherford explained the results of the experiment by proposing "planetary" model of the atom, which likened it to the solar system. According to the planetary model, at the center of the atom there is a very small nucleus, the size of which is approximately 100,000 times smaller sizes the atom itself. This nucleus contains almost the entire mass of the atom and carries a positive charge. Electrons move around the nucleus, the number of which is determined by the charge of the nucleus. The external trajectory of electron motion determines external dimensions atom. The diameter of an atom is on the order of 10 -8 cm, and the diameter of the nucleus is on the order of 10 -13 ÷10 -12 cm.
The more charge atomic nucleus, the stronger it will push away from it α -particle, the more often cases of strong deviations will occur α -particles passing through the metal layer, from the initial direction of movement. Therefore, scattering experiments α -particles make it possible not only to detect the existence of an atomic nucleus, but also to determine its charge. Already from Rutherford's experiments it followed that the charge of the nucleus (expressed in units of electron charge) is numerically equal to the serial number of the element in the periodic table. This has been confirmed G. Moseley, who established in 1913 a simple connection between the wavelengths of certain lines in the X-ray spectrum of an element and its atomic number, and D. Chadwick, who in 1920 determined with great accuracy the charges of atomic nuclei of a number of elements by scattering α -particles
The physical meaning of the serial number of an element in the periodic system was established: the serial number turned out to be the most important constant of an element, expressing the positive charge of the nucleus of its atom. From the electrical neutrality of an atom it follows that the number of electrons rotating around the nucleus is equal to the atomic number of the element.
This discovery provided a new rationale for the arrangement of elements in the periodic table. At the same time, it also eliminated the apparent contradiction in the Mendeleev system - the position of some elements with higher atomic mass ahead of elements with lower atomic mass (tellurium and iodine, argon and potassium, cobalt and nickel). It turned out that there is no contradiction here, since the place of an element in the system is determined by the charge of the atomic nucleus. It was experimentally established that the nuclear charge of a tellurium atom is 52, and that of an iodine atom is 53; therefore tellurium, despite the large atomic mass, must stand before iodine. In the same way, the charges of the nuclei of argon and potassium, nickel and cobalt fully correspond to the sequence of arrangement of these elements in the system.
So, the charge of the atomic nucleus is the basic quantity on which the properties of the element and its position in the periodic table depend. That's why periodic law of mendeleev can currently be formulated as follows:
The properties of elements and the simple and complex substances they form are periodically dependent on the charge of the nucleus of the atoms of the elements
Determining the serial numbers of elements based on the charges of the nuclei of their atoms made it possible to establish total number places in the periodic table between hydrogen, which has serial number 1, and uranium (atomic number 92), which was considered at that time the last member of the periodic table of elements. When the theory of atomic structure was created, places 43, 61, 72, 75, 85 and 87 remained unoccupied, which indicated the possibility of the existence of as yet undiscovered elements. Indeed, in 1922 the element hafnium was discovered, which took place 72; then in 1925 - rhenium, which took place 75. Elements that should occupy the remaining four free seats tables turned out to be radioactive and were not found in nature, but they were obtained artificially. The new elements were named technetium (serial number 43), promethium (61), astatine (85) and francium (87). Currently, all the cells of the periodic table between hydrogen and uranium are filled. However, she herself periodic table is not complete.
Atomic spectra
The planetary model was a major step in the theory of atomic structure. However, in some respects it contradicted firmly established facts. Let's consider two such contradictions.
First, Rutherford's theory could not explain the stability of the atom. An electron orbiting a positively charged nucleus should, like an oscillating electric charge, emit electromagnetic energy in the form of light waves. But by emitting light, the electron loses part of its energy, which leads to an imbalance between the centrifugal force associated with the rotation of the electron and the force of electrostatic attraction of the electron to the nucleus. To restore equilibrium, the electron must move closer to the nucleus. Thus, the electron, continuously emitting electromagnetic energy and moving in a spiral, will approach the nucleus. Having exhausted all its energy, it must “fall” onto the nucleus, and the atom will cease to exist. This conclusion contradicts the real properties of atoms, which are stable formations and can exist without destruction for an extremely long time.
Secondly, Rutherford's model led to incorrect conclusions about the nature of atomic spectra. When light emitted by a hot solid or liquid body is passed through a glass or quartz prism, a so-called continuous spectrum is observed on a screen placed behind the prism, the visible part of which is a colored stripe containing all the colors of the rainbow. This phenomenon is explained by the fact that the radiation of a hot solid or liquid body consists of electromagnetic waves of various frequencies. Waves of different frequencies are refracted differently by the prism and fall on different places screen. Set of frequencies electromagnetic radiation
emitted by a substance is called the emission spectrum. On the other hand, substances absorb radiation of certain frequencies. The combination of the latter is called the absorption spectrum of the substance.
When heated, a substance emits rays (radiation). If radiation has one wavelength, then it is called monochromatic. In most cases, radiation is characterized by several wavelengths.
When the radiation is decomposed into monochromatic components, a radiation spectrum is obtained, where its individual components are expressed as spectral lines.
Spectra obtained by emission from free or weakly bound atoms (for example, in gases or vapors) are called atomic spectra.
Radiation emitted by solids or liquids always gives a continuous spectrum. Radiation emitted by hot gases and vapors, unlike radiation from solids and liquids, contains only certain wavelengths. Therefore, instead of a continuous stripe on the screen, you get a series of individual colored lines separated by dark spaces. The number and location of these lines depend on the nature of the hot gas or steam. Thus, potassium vapor produces a spectrum consisting of three lines - two red and one violet; in the spectrum of calcium vapor there are several red, yellow and green lines, etc.
Radiation emitted by solids or liquids always gives a continuous spectrum. Radiation emitted by hot gases and vapors, unlike radiation from solids and liquids, contains only certain wavelengths. Therefore, instead of a continuous stripe on the screen, you get a series of individual colored lines separated by dark spaces. The number and location of these lines depend on the nature of the hot gas or steam. Thus, potassium vapor gives a spectrum consisting of three lines - two red and one violet; in the spectrum of calcium vapor there are several red, yellow and green lines, etc.
Such spectra are called line spectra. It was found that light emitted by gas atoms has a line spectrum, in which spectral lines can be combined into series. In each series, the arrangement of lines corresponds to a certain pattern. The frequencies of individual lines can be described:
Balmer's formula The fact that the atoms of each element give a completely definite spectrum, inherent only to this element, and the intensity of the corresponding spectral lines is higher, the higher the content of the element in the sample taken, is widely used to determine the qualitative and quantitative composition of substances and materials. This research method is called.
The planetary model of the structure of the atom turned out to be unable to explain the line spectrum of emission of hydrogen atoms, much less the combination of spectral lines in a series. An electron rotating around a nucleus must approach the nucleus, continuously changing its speed. The frequency of the light it emits is determined by the frequency of its rotation and therefore must change continuously. This means that the emission spectrum of an atom must be continuous, continuous. According to this model, the frequency of radiation of an atom must be equal to the mechanical frequency of vibrations or be a multiple of it, which does not agree with Balmer’s formula.
Thus, Rutherford's theory could not explain either the existence of stable atoms or the presence of their line spectra.
Quantum theory of light In 1900 M. Planck showed that the ability of a heated body to emit radiation can be correctly described quantitatively only by assuming that radiant energy is emitted and absorbed by bodies not continuously, but discretely, i.e. in separate portions - quanta. At the same time, the energy E each such portion is related to the radiation frequency by a relationship called:
Planck's equations Planck himself for a long time believed that the emission and absorption of light by quanta is a property of emitting bodies, and not the radiation itself, which is capable of having any energy and therefore could be absorbed continuously. However, in 1905 Einstein , analyzing the phenomenon of the photoelectric effect, came to the conclusion that electromagnetic (radiant) energy exists only in the form of quanta and that, therefore, radiation is a stream of indivisible material “particles” (photons), the energy of which is determined by.

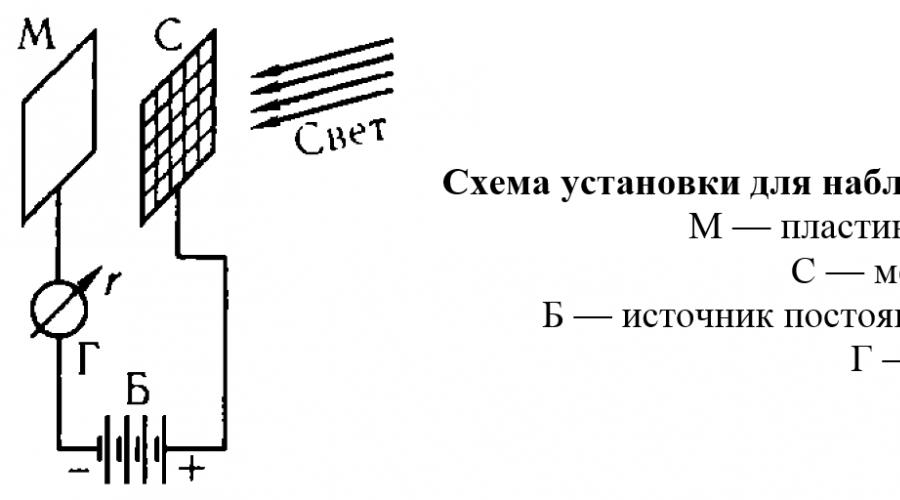
Planck's equation Photoelectric effect is the emission of electrons by a metal under the influence of light incident on it. This phenomenon was studied in detail in 1888-1890. A. G. Stoletov . If you place the installation in a vacuum and apply it to a record M negative potential
, then no current will be observed in the circuit, since in the space between the plate and the grid there are no charged particles capable of carrying electric current. But when the plate is illuminated by a light source, the galvanometer detects the emergence of a current (called photocurrent), the carriers of which are electrons emitted from the metal by light. It turned out that when the lighting intensity changes, only the number of electrons emitted by the metal changes, i.e. photocurrent strength. But the maximum each electron emitted from the metal does not depend on the intensity of illumination, but changes only when the frequency of the light incident on the metal changes. It is with an increase in wavelength (i.e., with a decrease in frequency) that the energy of the electrons emitted by the metal decreases, and then, at a wavelength specific to each metal, the photoelectric effect disappears and does not appear even at very high light intensity. Thus, when illuminated with red or orange light, sodium does not exhibit a photoelectric effect and begins to emit electrons only at a wavelength less than 590 nm (yellow light); in lithium, the photoelectric effect is detected at even lower wavelengths, starting from 516 nm (green light); and the ejection of electrons from platinum under the influence of visible light does not occur at all and begins only when platinum is irradiated with ultraviolet rays.
These properties of the photoelectric effect are completely inexplicable from the standpoint of the classical wave theory of light, according to which the effect should be determined (for a given metal) only amount of energy, absorbed by the metal surface per unit time, but should not depend on the type of radiation incident on the metal. However, these same properties receive a simple and convincing explanation if we assume that the radiation consists of individual portions, photons, with a very specific energy.
In fact, an electron in a metal is bound to the metal atoms, so that a certain energy must be expended to tear it out. If the photon has the required amount of energy (and the energy of the photon is determined by the frequency of the radiation), then the electron will be ejected and the photoelectric effect will be observed. In the process of interacting with a metal, the photon completely gives up its energy to the electron, because the photon cannot be split into parts. The energy of the photon will be partially spent on breaking the bond between the electron and the metal, and partially on imparting kinetic energy of motion to the electron. Therefore, the maximum kinetic energy of an electron knocked out of a metal cannot be greater than the difference between the photon energy and the binding energy of the electron with the metal atoms. Consequently, with an increase in the number of photons incident on the metal surface per unit time (i.e., with an increase in illumination intensity), only the number of electrons ejected from the metal will increase, which will lead to an increase in the photocurrent, but the energy of each electron will not increase. If the photon energy is less than the minimum energy required to eject an electron, the photoelectric effect will not be observed for any number of photons incident on the metal, i.e. at any lighting intensity.
Quantum theory of light, developed Einstein, was able to explain not only the properties of the photoelectric effect, but also the patterns of the chemical action of light, temperature dependence heat capacities of solids and a number of other phenomena. It turned out to be extremely useful in the development of ideas about the structure of atoms and molecules.
From quantum theory light, it follows that the photon is incapable of fragmentation: it interacts as a whole with the electron of the metal, knocking it out of the plate; as a whole, it interacts with the light-sensitive substance of the photographic film, causing it to darken at a certain point, etc. In this sense, the photon behaves like a particle, i.e. exhibits corpuscular properties. However, the photon also has wave properties: this is manifested in the wave nature of the propagation of light, in the photon’s ability to interfere and diffraction. A photon differs from a particle in the classical sense of the term in that its exact position in space, like the exact position of any wave, cannot be specified. But it also differs from the “classical” wave in its inability to divide into parts. Combining corpuscular and wave properties, the photon is, strictly speaking, neither a particle nor a wave - it is characterized by corpuscular-wave duality.
Lecture: Planetary model of the atom
Atomic structure
The most accurate way to determine the structure of any substance is spectral analysis. The radiation of each atom of an element is exclusively individual. However, before we understand how spectral analysis occurs, we will understand what structure an atom of any element has.
The first assumption about the structure of the atom was presented by J. Thomson. This scientist long time studied atoms. Moreover, it was he who discovered the electron - for which he received Nobel Prize. The model that Thomson proposed had nothing to do with reality, but it served as a fairly strong stimulus in Rutherford’s study of the structure of the atom. The model proposed by Thomson was called "raisin pudding".

Thomson believed that the atom was a solid ball with a negative electric charge. To compensate for this, electrons are interspersed into the ball, like raisins. The total charge of the electrons coincides with the charge of the entire nucleus, which makes the atom neutral.
While studying the structure of the atom, they found out that all atoms in solids commit oscillatory movements. And, as you know, any moving particle emits waves. This is why each atom has its own spectrum. However, these statements were not included in Thomson’s model in any way.
Rutherford's experience
To confirm or refute Thomson's model, Rutherford proposed an experiment in which an atom of a certain element was bombarded with alpha particles. As a result of this experiment, it was important to see how the particle would behave.
Alpha particles were discovered as a result of the radioactive decay of radium. Their streams were alpha rays, each particle of which had a positive charge. As a result of numerous studies, it was determined that the alpha particle is like a helium atom, which lacks electrons. Using current knowledge we know that the alpha particle is a helium nucleus, at the time Rutherford believed it was helium ions.
Each alpha particle had enormous energy, as a result of which it could fly towards the atoms in question at high speeds. Therefore, the main result of the experiment was to determine the angle of deflection of the particle.
To carry out the experiment, Rutherford used thin foil made of gold. He directed high-speed alpha particles at her. He assumed that as a result of this experiment, all particles would fly through the foil, and with slight deviations. However, to find out for sure, he instructed his students to check whether these particles had large deviations.

The result of the experiment surprised absolutely everyone, because many particles not only deviated by a fairly large angle - some deviation angles reached more than 90 degrees.
These results surprised absolutely everyone; Rutherford said that it felt as if a piece of paper was placed in the path of the projectiles, which did not allow the alpha particle to penetrate inside, as a result of which it turned back.
If the atom were really solid, then it should have some electric field, which slowed down the particle. However, the strength of the field was not enough to stop it completely, much less throw it back. This means that Thomson's model has been refuted. So Rutherford began working on a new model.
Rutherford model
To obtain such an experimental result, it is necessary to concentrate the positive charge in a smaller size, resulting in a larger electric field. Using the field potential formula, one can determine required size a positive particle that could push an alpha particle in the opposite direction. Its radius should be about maximum 10 -15 m. That is why Rutherford proposed the planetary model of the atom.

This model is named so for a reason. The fact is that inside the atom there is a positively charged nucleus, similar to the Sun in the solar system. Electrons revolve around the nucleus, like planets. The solar system is designed in such a way that the planets are attracted to the Sun by gravitational forces, however, they do not fall to the surface of the Sun as a result of the existing speed that keeps them in their orbit. The same thing happens with electrons - Coulomb forces attract electrons to the nucleus, but due to rotation they do not fall to the surface of the nucleus.
One of Thomson's assumptions turned out to be absolutely correct - the total charge of the electrons corresponds to the charge of the nucleus. However, as a result of strong interactions, electrons can be knocked out of their orbit, as a result of which the charge is not compensated and the atom turns into a positively charged ion.
Very important information Regarding the structure of the atom is that almost the entire mass of the atom is concentrated in the nucleus. For example, a hydrogen atom has only one electron, whose mass is more than one and a half thousand times less than the mass of the nucleus.
| | |
Historical models1 of the atom reflect levels of knowledge corresponding to a certain period in the development of science.
The first stage of development of atomic models was characterized by the absence of experimental data on its structure.
Explaining the phenomena of the microworld, scientists looked for analogies in the macroworld, relying on the laws of classical mechanics.
J. Dalton, the creator of chemical atomism (1803), assumed that atoms of the same chemical element are identical spherical tiny, and therefore indivisible particles.
The French physicist Jean Baptiste Perrin (1901) proposed a model that actually anticipated the “planetary” model. According to this model, in the center of the atom there is a positively charged nucleus, around which negatively charged electrons move in certain orbits, like planets around the Sun. Perrin's model did not attract the attention of scientists, since it gave only a qualitative, but not a quantitative characteristic of the atom (in Fig. 7 this is shown by the discrepancy between the charge of the atomic nucleus and the number of electrons).
In 1902, the English physicist William Thomson (Kelvin) developed the idea of an atom as a positively charged spherical particle within which negatively charged electrons oscillate (emitting and absorbing energy). Kelvin drew attention to the fact that the number of electrons is equal to the positive charge of the sphere, therefore, as a whole, the atom has no electric charge(Fig. 7).
A year later, German physicist Philipp Lenard proposed a model according to which the atom is a hollow sphere, inside which there are electric dipoles (dynamides). The volume occupied by these dipoles is significantly less than the volume of the sphere, and the main part of the atom turns out to be unfilled.
According to the ideas of the Japanese physicist Gontaro (Hantaro) Nagaoki (1904), at the center of the atom there is a positively charged nucleus, and electrons move in space around the nucleus in flat rings, reminiscent of the rings of the planet Saturn (this model was called the “Saturnian” atom). Most scientists have not paid attention to Nagaoka's ideas, although they have some overlap with the modern idea of atomic orbital.
None of the models considered (Fig. 7) explained how the properties chemical elements associated with the structure of their atoms.
Rice. 7. Some historical models of the atom
In 1907, J. J. Thomson proposed a static model of the structure of the atom, which represented the atom as a spherical particle charged with positive electricity, in which negatively charged electrons are evenly distributed ( model"pudding", Fig. 7).
Mathematical calculations showed that the electrons in an atom should be located on concentrically located rings. Thomson made a very important conclusion: the reason for the periodic changes in the properties of chemical elements is associated with the peculiarities of the electronic structure of their atoms. Thanks to this, Thomson's atomic model was highly appreciated by his contemporaries. However, it did not explain some phenomena, for example, the scattering of α-particles when they pass through a metal plate.
Based on his ideas about the atom, Thomson derived a formula for calculating the average deviation of α-particles, and this calculation showed that the probability of scattering of such particles at large angles is close to zero. However, it has been experimentally proven that approximately one in eight thousand α-particles falling on gold foil is deflected by an angle greater than 90°. This contradicted Thomson's model, which assumed deviations only at small angles.
Ernest Rutherford, summarizing experimental data, in 1911 proposed a “planetary” (sometimes called “nuclear”) model of the structure of the atom, according to which 99.9% of the mass of the atom and its positive charge are concentrated in a very small nucleus, and negatively charged electrons, the number which is equal to the charge of the nucleus, rotate around it, like planets solar system 1 (Fig. 7).
Rutherford, together with his students, conducted experiments that made it possible to study the structure of the atom (Fig. 8). A stream of positively charged particles (α-particles) was directed onto the surface of thin metal (gold) foil 2 from a radioactive radiation source 1. A fluorescent screen 3 was installed along their path, making it possible to observe the direction of further movement of α-particles.

Rice. 8. Rutherford's experiment
It was found that the majority of α-particles passed through the foil, practically without changing their direction. Only a few particles (on average one in ten thousand) were deflected and flew almost in the opposite direction. It was concluded that most of the mass of the atom is concentrated in the positively charged nucleus, which is why α particles are deflected so much (Fig. 9).

Rice. 9. Scattering of α-particles by an atomic nucleus
Electrons moving in an atom, in accordance with the laws of electromagnetism, must emit energy and, losing it, be attracted to an oppositely charged nucleus and, therefore, “fall” onto it. This should lead to the disappearance of the atom, but since this did not happen, it was concluded that this model was inadequate.
At the beginning of the 20th century, the German physicist Max Planck and theoretical physicist Albert Einstein created the quantum theory of light. According to this theory, radiant energy, such as light, is emitted and absorbed not continuously, but in separate portions (quanta). Moreover, the magnitude of the energy quantum is not the same for different radiations and is proportional to the oscillation frequency electromagnetic wave: E = hν, whereh – Planck's constant, equal to 6.6266·10 –34 J·s, ν – radiation frequency. This energy is carried by particles of light - photons.
Trying to artificially combine the laws of classical mechanics and quantum theory, the Danish physicist Niels Bohr in 1913 supplemented the Rutherford model of the atom with two postulates about an abrupt (discrete) change in the energy of electrons in an atom. Bohr believed that an electron in a hydrogen atom can only be located at very specific positions. stationary orbits, whose radii are related to each other as squares natural numbers (1 2: 2 2: 3 2: ... :n 2). Electrons move around the atomic nucleus in stationary orbits. The atom remains in a stable state, neither absorbing nor emitting energy - this is Bohr's first postulate. According to the second postulate, energy emission occurs only when an electron moves to an orbit closer to the atomic nucleus. When an electron moves to a more distant orbit, energy is absorbed by the atom. This model was improved in 1916 by the German theoretical physicist Arnold Sommerfeld, who pointed out the movement of electrons along elliptical orbits.
The planetary model, due to its clarity and Bohr's postulates, has been used for a long time to explain atomic and molecular phenomena. However, it turned out that the movement of an electron in an atom, the stability and properties of the atom, in contrast to the movement of planets and the stability of the Solar system, cannot be described by the laws of classical mechanics. This mechanics is based on Newton's laws, and the subject of its study is the movement of macroscopic bodies at speeds small compared to the speed of light. To describe the structure of the atom, it is necessary to apply the concepts of quantum (wave) mechanics about the dual corpuscular-wave nature of microparticles, which were formulated in the 1920s by theoretical physicists: the Frenchman Louis de Broglie, the Germans Werner Heisenberg and Erwin Schrödinger, the Englishman Paul Dirac, etc.
In 1924, Louis de Broglie hypothesized that the electron has wave properties (first principle quantum mechanics) and proposed a formula for calculating its wavelength. The stability of an atom is explained by the fact that the electrons in it do not move in orbits, but in certain regions of space around the nucleus, called atomic orbitals. An electron occupies almost the entire volume of an atom and cannot “fall onto the nucleus” located at its center.
In 1926, Schrödinger, continuing the development of L. de Broglie’s ideas about the wave properties of the electron, empirically selected a mathematical equation similar to the equation of string vibration, with the help of which one can calculate the binding energies of an electron in an atom at different energy levels. This equation became the fundamental equation of quantum mechanics.
The discovery of the wave properties of the electron showed that the spread of knowledge about the macroworld to objects of the microworld is unlawful. In 1927, Heisenberg established that it is impossible to determine the exact position in space of an electron having a certain speed, therefore ideas about the movement of an electron in an atom are probabilistic in nature (the second principle of quantum mechanics).
The quantum mechanical model of the atom (1926) describes the state of the atom through mathematical functions and does not have a geometric expression (Fig. 10). This model does not consider the dynamic nature of the atomic structure and the question of the size of the electron as a particle. Electrons are believed to occupy certain energy levels and emit or absorb energy when moving to other levels. In Fig. 10 energy levels are depicted schematically in the form of concentric rings located at different distances from the atomic nucleus. The arrows indicate transitions of electrons between energy levels and the emission of photons accompanying these transitions. The diagram is shown qualitatively and does not reflect the real distances between energy levels, which can differ from each other by tens of times.
In 1931, the American scientist Gilbert White first proposed a graphical representation of atomic orbitals and an “orbital” model of the atom (Fig. 10). Atomic orbital models are used to reflect the concept of electron density and show the distribution of negative charge around a nucleus in an atom or a system of atomic nuclei in a molecule.

Rice. 10. Historical and modern models atom
In 1963, the American artist, sculptor and engineer Kenneth Snelson proposed a “ring-edge model” of the electron shells of an atom (Fig. 10), which explains the quantitative distribution of electrons in an atom across stable electron shells. Each electron is modeled as a ring magnet (or a closed loop with an electric current that has a magnetic moment). Ring magnets attract each other and form symmetrical ring shapes - annulus. The presence of two poles in magnets imposes a limitation on possible options ring-shaped assemblies. Models of stable electron shells are the most symmetrical figures of rings, composed taking into account their magnetic properties.
The presence of spin in an electron (see Section 5) is one of the main reasons for the formation of stable electron shells in an atom. Electrons form pairs with opposite spins. The ring-sided model of an electron pair, or a filled atomic orbital, is two rings located in parallel planes on opposite sides of the atomic nucleus. When more than one pair of electrons is located near the nucleus of an atom, the ring electrons are forced to mutually orient themselves, forming an electron shell. In this case, closely spaced rings have different directions magnetic power lines, which is denoted different colors rings representing electrons.
The model experiment shows that the most stable of all possible ring-sided models is the model of 8 rings. Geometrically, the model is formed in such a way as if an atom in the form of a sphere was divided into 8 parts (divided in half three times) and one ring-electron was placed in each part. Ring-edge models use rings of two colors: red and blue, which reflect the positive and negative spin of the electron.
The “wave-faceted model” (Fig. 10) is similar to the “ring-faceted” one with the difference that each electron of the atom is represented by a “wave” ring that contains an integer number of waves (as proposed by L. de Broglie).
The interaction of electrons in the electron shell on this atomic model is shown by the coincidence of the contact points of the blue and red “wave” rings with the nodes of standing waves.
Atomic models have a right to exist and limits of application. Any model of an atom is an approximation that reflects in a simplified form a certain part of knowledge about the atom. But none of the models fully reflects the properties of the atom or its constituent particles.
Many models today are of historical interest only. When constructing models of objects in the microworld, scientists relied on what could be directly observed. This is how the models of Perrin and Rutherford (an analogy with the structure of the solar system), Nagaoka (something like the planet Saturn), and Thomson ("raisin pudding") appeared. Some ideas were discarded (Lenard's dynamic model), others were revisited after some time, but at a new, higher theoretical level: the Perrin and Kelvin models were developed in the Rutherford and Thomson models. Ideas about the structure of the atom are constantly being improved. Time will tell how accurate the modern “quantum mechanical” model is. That is why a question mark is drawn at the top of the spiral, symbolizing the path of knowledge (Fig. 7).
The idea that atoms are the smallest particles of matter first arose in ancient Greece. However, only in late XVIII century, thanks to the work of such scientists as A. Lavoisier, M.V. Lomonosov and some others, it was proven that atoms really exist. However, in those days no one wondered what their internal structure was. Scientists still regarded atoms as the indivisible “building blocks” that make up all matter.
Attempts to explain the structure of the atom
Who was the first scientist to propose the nuclear model? The first attempt to create a model of these particles belonged to J. Thomson. However, it cannot be called successful in the full sense of the word. After all, Thomson believed that the atom is a spherical and electrically neutral system. At the same time, the scientist assumed that the positive charge was distributed evenly throughout the volume of this ball, and inside it there was a negatively charged nucleus. All the scientist’s attempts to explain the internal structure of the atom were unsuccessful. Ernest Rutherford is the one who proposed the nuclear model of the structure of the atom a few years after Thomson put forward his theory.

History of research
By researching electrolysis in 1833, Faraday was able to establish that the current in an electrolyte solution is a flow of charged particles, or ions. Based on these studies, he was able to determine the minimum charge of the ion. Also, an important role in the development of this direction in physics was played by the domestic chemist D.I. Mendeleev. It was he who first raised the question in scientific circles that all atoms could have the same nature. We see that before the Rutherford nuclear model of the structure of the atom was first proposed, a large number of equally important experiments were carried out by a variety of scientists. They advanced the atomic theory of the structure of matter.

First experiments
Rutherford is truly a brilliant scientist, because his discoveries revolutionized the understanding of the structure of matter. In 1911, he was able to set up an experiment, with the help of which researchers were able to look into the mysterious depths of the atom and get an idea of what its internal structure is. The first experiments were carried out by the scientist with the support of other researchers, however the main role in the opening it still belonged to Rutherford.

Experiment
Using natural springs radioactive radiation, Rutherford was able to build a gun that emitted a stream of alpha particles. It was a box made of lead, inside of which there was a radioactive substance. There was a slot in the gun that allowed all the alpha particles to hit the lead screen. They could only fly out through the slot. In the path of this beam of radioactive particles there were several more screens.
They separated particles that deviated from a previously specified direction. A strictly focused one hit the target. Rutherford used as a target thin sheet from gold foil. Once the particles hit this sheet, they continued their movement and eventually hit a fluorescent screen that was installed behind this target. When alpha particles hit this screen, flashes were recorded, from which the scientist could judge how many particles deviated from the original direction when colliding with the foil and what the magnitude of this deviation was.

Differences from previous experiments
Schoolchildren and students who are interested in who proposed the nuclear model of the structure of the atom should know: similar experiments were carried out in physics before Rutherford. Their main idea was to collect as much as possible from the deviations of particles from the initial trajectory more information about the structure of the atom. All these studies led to the accumulation of a certain amount of information in science and provoked thinking about internal structure smallest particles.
Already at the beginning of the 20th century, scientists knew that an atom contains electrons with a negative charge. But among most researchers, the prevailing opinion was that the inside of an atom is more like a grid filled with negatively charged particles. Similar experiences allowed us to obtain a lot of information - for example, to determine geometric dimensions atoms.
Brilliant guess
Rutherford noticed that none of his predecessors had ever tried to determine whether alpha particles could deviate at very large angles from their trajectory. The previous model, sometimes called “raisin pudding” among scientists (because according to this model, the electrons in an atom are distributed like raisins in a pudding), simply did not allow for the existence of dense components of the structure within the atom. None of the scientists even bothered to consider this option. The researcher asked his student to re-equip the installation in such a way that large deviations of particles from the trajectory were recorded - only to exclude this possibility. Imagine the surprise of both the scientist and his student when it turned out that some particles scatter at an angle of 180 degrees.
What's inside an atom?
We found out who proposed the nuclear model of the structure of the atom and what the experience of this scientist was. At that time, Rutherford's experiment was a real breakthrough. He was forced to conclude that inside an atom, most of the mass was contained in very dense matter. The diagram of the nuclear model of the structure of an atom is extremely simple: inside there is a positively charged nucleus.
Other particles called electrons orbit around this nucleus. The rest is several orders of magnitude less dense. The arrangement of electrons inside an atom is not chaotic - the particles are arranged in order of increasing energy. The researcher called the internal parts of atoms nuclei. The names that the scientist introduced are still used in science today.
How to prepare for the lesson?
Those schoolchildren who are interested in who proposed the nuclear model of the structure of the atom can show off additional knowledge in the lesson. For example, you can talk about how Rutherford, long after his experiments, liked to give an analogy for his discovery. Guns for rebels are being smuggled into a southern African country, contained in bales of cotton. How can customs officers determine exactly where dangerous supplies are located if the entire train is filled with these bales? The customs officer may start shooting at the bales, and where the bullets will ricochet is where the weapon is located. Rutherford emphasized that this is exactly how his discovery was made.
For schoolchildren who are preparing to answer on this topic in class, it is advisable to prepare answers to the following questions:
1. Who proposed the nuclear model of the structure of the atom?
2. What was the point of the experiment?
3. Difference between the nuclear model and other models.

The significance of Rutherford's theory
The radical conclusions that Rutherford drew from his experiments led many of his contemporaries to doubt the truth of this model. Even Rutherford himself was no exception - he published the results of his research only two years after the discovery. Taking as a basis the classical ideas of how microparticles move, he proposed a nuclear planetary model of the structure of the atom. Overall, the atom has a neutral charge. Electrons move around the nucleus, just as planets revolve around the Sun. This movement occurs due to Coulomb forces. At the moment, Rutherford's model has undergone significant modification, but the scientist's discovery does not lose its relevance today.
The planetary model of the atom was proposed by E. Rutherford in 1910. He made his first studies of the structure of the atom using alpha particles. Based on the results obtained from their scattering experiments, Rutherford proposed that all the positive charge of an atom was concentrated in a tiny nucleus at its center. On the other hand, negatively charged electrons are distributed throughout the rest of its volume.
A little background
The first brilliant guess about the existence of atoms was made by the ancient Greek scientist Democritus. Since then, the idea of the existence of atoms, the combinations of which give rise to all the substances around us, has not left the imagination of people of science. Various of its representatives periodically addressed it, but until the beginning of the 19th century, their constructions were just hypotheses, not supported by experimental data.
Finally, in 1804, more than a hundred years before the planetary model of the atom appeared, the English scientist John Dalton presented evidence of its existence and introduced the concept of atomic weight, which was its first quantitative characteristic. Like his predecessors, he conceived of atoms as tiny pieces of matter, like solid balls that could not be divided into even smaller particles.
Discovery of the electron and the first model of the atom
Almost a century passed when, finally, late XIX century also the Englishman J. J. Thomson discovered the first subatomic particle, a negatively charged electron. Since atoms are electrically neutral, Thomson thought that they must consist of a positively charged nucleus with electrons scattered throughout its volume. Based on different results obtained experimentally, in 1898 he proposed his model of the atom, sometimes called the “plums in the pudding,” because the atom in it was represented as a sphere filled with some positively charged liquid into which electrons were embedded, like “plums in the pudding.” The radius of such a spherical model was about 10 -8 cm. The overall positive charge of the liquid is symmetrically and evenly balanced by the negative charges of electrons, as shown in the figure below.
This model satisfactorily explained the fact that when a substance is heated, it begins to emit light. Although this was the first attempt to understand what an atom was, it failed to satisfy the results of experiments carried out later by Rutherford and others. Thomson agreed in 1911 that his model simply could not answer how and why the experimentally observed scattering of α-rays occurs. Therefore, it was abandoned, and was replaced by a more advanced planetary model of the atom.
How is the atom structured?
Ernest Rutherford provided an explanation of the phenomenon of radioactivity that won him the Nobel Prize, but his most significant contribution to science came later when he established that the atom consists of a dense nucleus surrounded by orbits of electrons, just as the Sun is surrounded by the orbits of planets.

According to the planetary model of the atom, most of its mass is concentrated in a tiny (compared to the size of the entire atom) nucleus. Electrons move around the nucleus, traveling at incredible speeds, but most of the volume of the atoms is empty space.
The size of the nucleus is so small that its diameter is 100,000 times smaller than that of an atom. The diameter of the nucleus was estimated by Rutherford to be 10 -13 cm, in contrast to the size of the atom - 10 -8 cm. Outside the nucleus, electrons rotate around it with high speeds, resulting in centrifugal forces that balance the electrostatic forces of attraction between protons and electrons.
Rutherford's experiments
The planetary model of the atom arose in 1911, after famous experiment with gold foil, which made it possible to obtain some fundamental information about its structure. Rutherford's path to the discovery of the atomic nucleus is good example the role of creativity in science. His search began back in 1899, when he discovered that some elements emit positively charged particles that can penetrate anything. He called these particles alpha (α) particles (we now know that they were helium nuclei). Like all good scientists, Rutherford was curious. He wondered if alpha particles could be used to learn the structure of an atom. Rutherford decided to aim a beam of alpha particles at a sheet of very thin gold foil. He chose gold because it could be made into sheets as thin as 0.00004 cm. Behind a sheet of gold foil, he placed a screen that glowed when alpha particles hit it. It was used to detect alpha particles after they passed through foil. A small slit in the screen allowed the alpha particle beam to reach the foil after leaving the source. Some of them should pass through the foil and continue to move in the same direction, the other part should bounce off the foil and be reflected under sharp corners. You can see the experimental design in the figure below.

What happened in Rutherford's experiment?
Based on J. J. Thomson's model of the atom, Rutherford assumed that continuous regions of positive charge filling the entire volume of gold atoms would deflect or bend the trajectories of all alpha particles as they passed through the foil.
However, the vast majority of alpha particles passed straight through the gold foil, as if it were not there. They seemed to be passing through empty space. Only a few of them deviate from the straight path, as expected at the beginning. Below is a graph of the number of particles scattered in the corresponding direction versus the scattering angle.

Surprisingly, a tiny percentage of the particles bounced back off the foil, like a basketball bouncing off a backboard. Rutherford realized that these deviations were the result of direct collisions between alpha particles and the positively charged components of the atom.
The core takes center stage
Based on the tiny percentage of alpha particles reflected from the foil, we can conclude that all the positive charge and almost all the mass of the atom is concentrated in one small area, and the rest of the atom is mostly empty space. Rutherford called the area of concentrated positive charge the nucleus. He predicted and soon discovered that it contained positively charged particles, which he called protons. Rutherford predicted the existence of neutral atomic particles called neutrons, but he was unable to detect them. However, his student James Chadwick discovered them a few years later. The figure below shows the structure of the nucleus of a uranium atom.

Atoms consist of positively charged heavy nuclei surrounded by negatively charged extremely light electron particles rotating around them, and at such speeds that mechanical centrifugal forces simply balance their electrostatic attraction to the nucleus, and in this regard, supposedly, the stability of the atom is ensured.
Disadvantages of this model
Rutherford's main idea related to the idea of a small atomic nucleus. The assumption about electron orbits was pure hypothesis. He did not know exactly where and how the electrons revolved around the nucleus. Therefore, Rutherford's planetary model does not explain the distribution of electrons in orbits.
In addition, the stability of the Rutherford atom was possible only with the continuous movement of electrons in orbits without loss of kinetic energy. But electrodynamic calculations have shown that the movement of electrons along any curvilinear trajectories, accompanied by a change in the direction of the velocity vector and the appearance of a corresponding acceleration, is inevitably accompanied by radiation electromagnetic energy. In this case, according to the law of conservation of energy, the kinetic energy of the electron should be very quickly spent on radiation, and it should fall onto the nucleus, as shown schematically in the figure below.

But this does not happen, since atoms are stable formations. A contradiction, typical for science, arose between the model of the phenomenon and experimental data.
From Rutherford to Niels Bohr
The next big step forward in atomic history occurred in 1913, when the Danish scientist Niels Bohr published a description of a more detailed model of the atom. It more clearly defined the places where electrons could be located. Although scientists would later develop more sophisticated atomic designs, Bohr's planetary model of the atom was basically correct, and much of it is still accepted today. She had many useful applications, for example, it is used to explain the properties of various chemical elements, the nature of their radiation spectrum and the structure of the atom. The planetary model and the Bohr model were the most important milestones that marked the emergence of a new direction in physics - the physics of the microworld. Bohr received the 1922 Nobel Prize in Physics for his contributions to our understanding of atomic structure.
What new did Bohr bring to the atomic model?
While still a young man, Bohr worked in Rutherford's laboratory in England. Since the concept of electrons was poorly developed in Rutherford's model, Bohr focused on them. As a result, the planetary model of the atom was significantly improved. Bohr's postulates, which he formulated in his article “On the Structure of Atoms and Molecules,” published in 1913, state:
1. Electrons can move around the nucleus only at fixed distances from it, determined by the amount of energy they have. He called these fixed levels energy levels or electron shells. Bohr imagined them as concentric spheres, with a nucleus at the center of each. In this case, electrons with lower energy will be found at more low levels, closer to the core. Those with more energy will be found at more high levels, further from the core.
2. If an electron absorbs a certain (quite certain for a given level) amount of energy, then it will jump to the next, higher energy level. Conversely, if he loses the same amount of energy, he will return back to his original level. However, an electron cannot exist at two energy levels.
This idea is illustrated by a drawing.

Energy portions for electrons
Bohr's atomic model is actually a combination of two different ideas: Rutherford's atomic model with electrons revolving around the nucleus (essentially the Bohr-Rutherford planetary model of the atom), and the German scientist Max Planck's idea of quantizing the energy of matter, published in 1901. A quantum (in plural- quanta) is minimum quantity energy that can be absorbed or emitted by a substance. It is a kind of step of discretizing the amount of energy.
If energy is compared to water and you want to add it to matter in the form of a glass, you cannot simply pour water in a continuous stream. Instead, you can add it in small quantities, such as a teaspoon. Bohr believed that if electrons can only absorb or lose fixed amounts of energy, then they must vary their energy only by those fixed amounts. Thus, they can only occupy fixed energy levels around the nucleus that correspond to quantized increments of their energy.
Thus, from Bohr’s model grows a quantum approach to explaining what the structure of the atom is. The planetary model and the Bohr model were unique steps from classical physics to quantum physics, which is the main tool in the physics of the microworld, including atomic physics.