Corrosion of screen pipes of steam boilers. Guidelines for preventing low-temperature corrosion of heating surfaces and boiler flues. Alkali brittleness of steel
Read also
A number of boiler houses use river and tap water with a low pH value and low hardness to feed heating networks. Additional treatment of river water at a waterworks usually leads to a decrease in pH, a decrease in alkalinity and an increase in the content of aggressive carbon dioxide. The appearance of aggressive carbon dioxide is also possible in connection schemes used for large heat supply systems with direct hot water supply (2000-3000 t/h). Softening water according to the Na-cationization scheme increases its aggressiveness due to the removal of natural corrosion inhibitors - hardness salts.
With poorly established water deaeration and possible increases in oxygen and carbon dioxide concentrations, due to the lack of additional protective measures in heat supply systems, thermal power equipment of thermal power plants is susceptible to internal corrosion.
When examining the make-up tract of one of the thermal power plants in Leningrad, the following data were obtained on the corrosion rate, g/(m2 4):
Installation location of corrosion indicators
In the make-up water pipeline after the heaters of the heating network in front of the deaerators, the 7 mm thick pipes thinned over the year of operation, in some places up to 1 mm, and through fistulas formed in some areas.
The causes of pitting corrosion of hot water boiler pipes are as follows:
insufficient removal of oxygen from make-up water;
low pH value due to the presence of aggressive carbon dioxide
(up to 10h15 mg/l);
accumulation of oxygen corrosion products of iron (Fe2O3;) on heat transfer surfaces.
Operating equipment on network water with an iron concentration of over 600 µg/l usually results in intensive (over 1000 g/m2) contamination of their heating surfaces with iron oxide deposits for several thousand hours of operation of hot water boilers. In this case, frequent leaks are noted in the pipes of the convective part. The content of iron oxides in sediments usually reaches 80–90%.
Start-up periods are especially important for the operation of hot water boilers. First initial period operation at one thermal power plant did not ensure oxygen removal to the standards established by the PTE. The oxygen content in the make-up water exceeded these standards by 10 times.
The concentration of iron in the make-up water reached 1000 µg/l, and in the return water of the heating network - 3500 µg/l. After the first year of operation, cuttings were made from the network water pipelines; it turned out that their surface contamination with corrosion products was over 2000 g/m2.
It should be noted that at this thermal power plant, before putting the boiler into operation, the internal surfaces of the screen pipes and convective beam pipes were subjected to chemical cleaning. By the time the samples of screen pipes were cut out, the boiler had worked for 5300 hours. The sample of the screen pipe had an uneven layer of black-brown iron oxide deposits, firmly bound to the metal; height of tubercles 10x12 mm; specific pollution 2303 g/m2.
Sediment composition, %
The metal surface under the layer of deposits was affected by ulcers up to 1 mm deep. Convective beam tubes with inside were covered with iron oxide type deposits of black-brown color with the height of the tubercles up to 3-4 mm. The surface of the metal under the deposits is covered with ulcers various sizes depth 0.3x1.2 and diameter 0.35x0.5 mm. Some tubes had through holes (fistulas).
When hot water boilers are installed in old district heating systems in which significant amounts of iron oxides have accumulated, cases of deposits of these oxides in the heated boiler pipes are observed. Before turning on the boilers, it is necessary to thoroughly flush the entire system.
A number of researchers recognize the important role in the occurrence of sub-sludge corrosion of the process of rusting of pipes of water heating boilers during their downtime, when proper measures have not been taken to prevent standstill corrosion. Foci of corrosion that occur under the influence of atmospheric air on wet surfaces of boilers, continue to function during boiler operation.
Identification of types of corrosion is difficult, and, therefore, errors are common in determining technologically and economically optimal measures to combat corrosion. The main necessary measures are taken in accordance with regulatory documents, which establish the limits of the main corrosion initiators.
GOST 20995-75 “Stationary steam boilers with pressure up to 3.9 MPa. Indicators of quality of feed water and steam" normalizes the indicators in feed water: transparency, that is, the amount of suspended impurities; general hardness, content of iron and copper compounds - prevention of scale formation and iron and copper oxide deposits; pH value - prevention of alkaline and acid corrosion and also foaming in the boiler drum; oxygen content - preventing oxygen corrosion; nitrite content - prevention of nitrite corrosion; content of petroleum products - preventing foam formation in the boiler drum.
The norm values are determined by GOST depending on the pressure in the boiler (therefore, on the water temperature), on the power of the local heat flow and on the water treatment technology.
When investigating the causes of corrosion, first of all, it is necessary to inspect (where available) places of metal destruction, analyze the operating conditions of the boiler in the pre-accident period, analyze the quality of feed water, steam and deposits, and analyze the design features of the boiler.
Upon external inspection, the following types of corrosion may be suspected.
Oxygen corrosion
: entrance areas steel economizer pipes; supply pipelines when encountering insufficiently deoxygenated (above normal) water - “breakthroughs” of oxygen due to poor deaeration; feed water heaters; all wet areas of the boiler during its shutdown and failure to take measures to prevent air from entering the boiler, especially in stagnant areas, when draining water, from where it is difficult to remove steam condensate or completely fill with water, for example, vertical pipes of superheaters. During downtime, corrosion is enhanced (localized) in the presence of alkali (less than 100 mg/l).
Oxygen corrosion rarely (when the oxygen content in water is significantly higher than the norm - 0.3 mg/l) appears in the steam separation devices of boiler drums and on the drum wall at the water level boundary; in downpipes. Corrosion does not occur in riser pipes due to the deaerating effect of steam bubbles.
Type and nature of damage. Ulcers of varying depth and diameter, often covered with tubercles, the upper crust of which is reddish iron oxides (probably hematite Fe 2 O 3). Evidence of active corrosion: under the crust of the tubercles there is a black liquid sediment, probably magnetite (Fe 3 O 4) mixed with sulfates and chlorides. With extinct corrosion, there is a void under the crust, and the bottom of the ulcer is covered with deposits of scale and sludge.
At water pH > 8.5 - ulcers are rare, but larger and deeper, at pH< 8,5 - встречаются чаще, но меньших размеров. Только вскрытие бугорков помогает интерпретировать бугорки не как поверхностные отложения, а как следствие коррозии.
When the water speed is more than 2 m/s, the tubercles can take on an oblong shape in the direction of the jet movement.
. Magnetic crusts are quite dense and could serve as a reliable barrier to the penetration of oxygen into the tubercles. But they are often destroyed as a result of corrosion fatigue, when the temperature of water and metal changes cyclically: frequent stops and starts of the boiler, pulsating movement of the steam-water mixture, stratification of the steam-water mixture into separate plugs of steam and water, following each other.
Corrosion increases with increasing temperature (up to 350 °C) and increasing chloride content in boiler water. Sometimes corrosion is enhanced by thermal decomposition products of certain organic substances in the feedwater.
Rice. 1. Appearance of oxygen corrosion
Alkaline (in a narrower sense - intergranular) corrosion
Places of metal corrosion damage. Pipes in heat flow zones high power(burner area and opposite the elongated torch) - 300-400 kW/m2 and where the metal temperature is 5-10 °C higher than the boiling point of water at a given pressure; inclined and horizontal pipes where water circulation is poor; places under thick sediments; zones near the backing rings and in the welds themselves, for example, in places where intra-drum vapor separation devices are welded; places near the rivets.
Type and nature of damage. Hemispherical or elliptical depressions filled with corrosion products, often including shiny crystals of magnetite (Fe 3 O 4). Most of the depressions are covered with a hard crust. On the side of the pipes facing the firebox, the recesses can connect, forming a so-called corrosion track 20-40 mm wide and up to 2-3 m long.
If the crust is not sufficiently stable and dense, then corrosion can lead - under conditions of mechanical stress - to the appearance of cracks in the metal, especially near the cracks: rivets, rolling joints, welding points of vapor separation devices.
Causes of Corrosion Damage. At high temperatures - more than 200 ° C - and high concentrations of sodium hydroxide (NaOH) - 10% or more - protective film(crust) on the metal is destroyed:
4NaOH + Fe 3 O 4 = 2NaFeO 2 + Na 2 FeO 2 + 2H 2 O (1)
The intermediate product NaFeO 2 undergoes hydrolysis:
4NaFeO 2 + 2H 2 O = 4NaOH + 2Fe 2 O 3 + 2H 2 (2)
That is, in this reaction (2) caustic soda is reduced, in reactions (1), (2) it is not consumed, but acts as a catalyst.
When the magnetite is removed, caustic soda and water can react with the iron directly to release atomic hydrogen:
2NaOH + Fe = Na 2 FeO 2 + 2H (3)
4H 2 O + 3Fe = Fe 3 O 4 + 8H (4)
The released hydrogen is able to diffuse into the metal and form methane (CH 4) with iron carbide:
4H + Fe 3 C = CH 4 + 3Fe (5)
It is also possible to combine atomic hydrogen into molecular hydrogen (H + H = H 2).
Methane and molecular hydrogen cannot penetrate into the metal; they accumulate at the grain boundaries and, in the presence of cracks, expand and deepen them. In addition, these gases prevent the formation and compaction of protective films.
A concentrated solution of caustic soda is formed in places of deep evaporation of boiler water: dense scale deposits of salts (a type of sub-sludge corrosion); a crisis of nucleate boiling, when a stable vapor film is formed above the metal - there the metal is almost not damaged, but at the edges of the film, where active evaporation occurs, caustic soda is concentrated; the presence of cracks where evaporation occurs, which is different from evaporation in the entire volume of water: caustic soda evaporates worse than water, is not washed away by water and accumulates. Acting on the metal, caustic soda forms cracks at the grain boundaries directed into the metal (a type of intergranular corrosion - crevice).
Intergranular corrosion under the influence of alkaline boiler water is most often concentrated in the boiler drum.

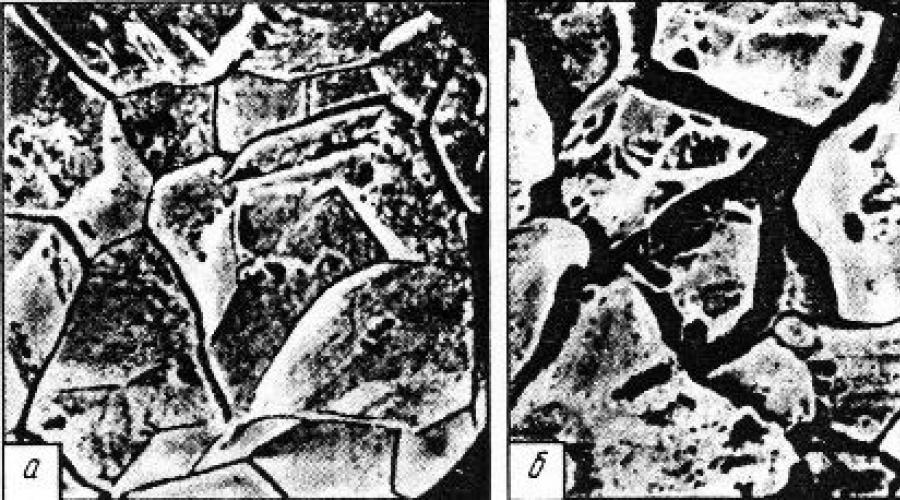
Rice. 3. Intergranular corrosion: a - microstructure of the metal before corrosion, b - microstructure at the corrosion stage, formation of cracks along the grain boundaries of the metal
Such a corrosive effect on metal is possible only with the simultaneous presence of three factors:
- local tensile mechanical stresses close to or slightly exceeding the yield strength, that is, 2.5 MN/mm 2 ;
- loose joints of drum parts (indicated above), where deep evaporation of boiler water can occur and where accumulating caustic soda dissolves the protective film of iron oxides (NaOH concentration is more than 10%, water temperature is above 200 ° C and - especially - closer to 300 ° C). If the boiler is operated at a pressure lower than the rated pressure (for example, 0.6-0.7 MPa instead of 1.4 MPa), then the likelihood of this type of corrosion decreases;
- an unfavorable combination of substances in boiler water, which lacks the necessary protective concentrations of inhibitors of this type of corrosion. Sodium salts can act as inhibitors: sulfates, carbonates, phosphates, nitrates, cellulose sulfite liquor.

Rice. 4. Appearance of intergranular corrosion
Corrosion cracks do not develop if the following ratio is observed:
(Na 2 SO 4 + Na 2 CO 3 + Na 3 PO 4 + NaNO 3)/(NaOH) ≥ 5.3 (6)
where Na 2 SO 4, Na 2 CO 3, Na 3 PO 4, NaNO 3, NaOH are the contents of sodium sulfate, sodium carbonate, sodium phosphate, sodium nitrate and sodium hydroxide, respectively, mg/kg.
In currently manufactured boilers, at least one of the specified conditions for the occurrence of corrosion is absent.
The presence of silicon compounds in boiler water can also increase intergranular corrosion.
NaCl under these conditions is not a corrosion inhibitor. It was shown above: chlorine ions (Cl -) are corrosion accelerators; due to their high mobility and small size, they easily penetrate protective oxide films and produce highly soluble salts with iron (FeCl 2, FeCl 3) instead of poorly soluble iron oxides.
In boiler water, the values of total mineralization are traditionally monitored, rather than the content of individual salts. Probably for this reason, standardization was introduced not according to the indicated ratio (6), but according to the value of the relative alkalinity of the boiler water:
Sh q rel = Sh ov rel = Sh ov 40 100/S ov ≤ 20, (7)
where Shk rel - relative alkalinity of boiler water, %; Shch ov rel - relative alkalinity of treated (additional) water, %; Shch ov - total alkalinity of treated (additional) water, mmol/l; S ov - mineralization of treated (additional) water (including chloride content), mg/l.
The total alkalinity of the treated (additional) water can be taken equal, mmol/l:
- after sodium cationization - the total alkalinity of the source water;
- after hydrogen-sodium cationization parallel - (0.3-0.4), or sequential with “hungry” regeneration of the hydrogen-cation exchange filter - (0.5-0.7);
- after sodium cationization with acidification and sodium chlorine ionization - (0.5-1.0);
- after ammonium-sodium cationization - (0.5-0.7);
- after liming at 30-40 °C - (0.35-1.0);
- after coagulation - (Sh about ref - D k), where Sh about ref is the total alkalinity of the source water, mmol/l; D k - dose of coagulant, mmol/l;
- after soda liming at 30-40 °C - (1.0-1.5), and at 60-70 °C - (1.0-1.2).
The values of relative alkalinity of boiler water according to Rostechnadzor standards are accepted, %, no more than:
- for boilers with riveted drums - 20;
- for boilers with welded drums and pipes rolled into them - 50;
- for boilers with welded drums and pipes welded to them - any value, not standardized.

Rice. 4. Result of intergranular corrosion
According to Rostekhnadzor standards, Shk kv rel is one of the criteria safe work boilers It is more correct to check the criterion for the potential alkaline aggressiveness of boiler water, which does not take into account the content of chlorine ion:
K sh = (S ov - [Cl - ])/40 Shch ov, (8)
where Ksh is a criterion for the potential alkaline aggressiveness of boiler water; S ov - mineralization of treated (additional) water (including chloride content), mg/l; Cl - - content of chlorides in treated (additional) water, mg/l; Shch ov - total alkalinity of treated (additional) water, mmol/l.
The value of K sch can be taken:
- for boilers with riveted drums pressure more than 0.8 MPa ≥ 5;
- for boilers with welded drums and pipes rolled into them with a pressure of more than 1.4 MPa ≥ 2;
- for boilers with welded drums and pipes welded to them, as well as for boilers with welded drums and pipes rolled into them with a pressure of up to 1.4 MPa and boilers with riveted drums with a pressure of up to 0.8 MPa - do not standardize.
Sludge corrosion
Under this name several different types corrosion (alkali, oxygen, etc.). The accumulation of loose and porous deposits and sludge in different areas of the boiler causes corrosion of the metal under the sludge. main reason: contamination of feed water with iron oxides.
Nitrite corrosion
. Screen and boiler pipes of the boiler on the side facing the firebox.
Type and nature of damage. Rare, sharply limited large ulcers.
. If there are more than 20 μg/l of nitrite ions (NO - 2) in the feed water, and the water temperature is more than 200 ° C, nitrites serve as cathodic depolarizers of electrochemical corrosion, being reduced to HNO 2, NO, N 2 (see above).
Steam-water corrosion
Locations of metal corrosion damage. The outlet part of superheater coils, superheated steam steam pipelines, horizontal and slightly inclined steam generating pipes in areas of poor water circulation, sometimes along the upper form of the outlet coils of boiling water economizers.
Type and nature of damage. Plaques of dense black iron oxides (Fe 3 O 4), firmly adhered to the metal. When the temperature fluctuates, the continuity of the plaque (crust) is disrupted and the scales fall off. Uniform thinning of metal with bulges, longitudinal cracks, breaks.
Can be identified as sub-slurry corrosion: in the form of deep ulcers with poorly demarcated edges, most often near pipes protruding inward welds where sludge accumulates.
Causes of corrosion damage:
- washing medium - steam in superheaters, steam pipelines, steam “pillows” under a layer of sludge;
- metal temperature (steel 20) more than 450 °C, heat flow to the metal section - 450 kW/m2;
- violation of the combustion regime: slagging of burners, increased contamination of pipes inside and outside, unstable (vibrating) combustion, elongation of the torch towards the screen pipes.
The result: direct chemical interaction of iron with water vapor (see above).
Microbiological corrosion
Caused by aerobic and anaerobic bacteria, appears at temperatures of 20-80 ° C.
Locations of metal damage. Pipes and containers to the boiler with water at the specified temperature.
Type and nature of damage. tubercles different sizes: diameter from several millimeters to several centimeters, rarely several tens of centimeters. The tubercles are covered with dense iron oxides - a waste product of aerobic bacteria. Inside there is a black powder and suspension (iron sulfide FeS) - a product of sulfate-reducing anaerobic bacteria; under the black formation there are round ulcers.
Causes of damage. Natural water always contains iron sulfates, oxygen and various bacteria.
Iron bacteria in the presence of oxygen form a film of iron oxides, under which anaerobic bacteria reduce sulfates to iron sulfide (FeS) and hydrogen sulfide (H 2 S). In turn, hydrogen sulfide starts the formation of sulfurous (very unstable) and sulfuric acids, and the metal corrodes.
This type has an indirect effect on boiler corrosion: a water flow at a speed of 2-3 m/s tears off the tubercles, carries their contents into the boiler, increasing the accumulation of sludge.
In rare cases, this corrosion may occur in the boiler itself if, during a long shutdown of the boiler, the reserve is filled with water at a temperature of 50-60 o C, and the temperature is maintained due to random breakthroughs of steam from neighboring boilers.
Chelate corrosion
Locations of corrosion damage. Equipment in which steam is separated from water: boiler drum, steam separation devices in and outside the drum, also - rarely - in feedwater pipelines and economizer.
Type and nature of damage. The surface of the metal is smooth, but if the medium moves at high speed, then the corroded surface is not smooth, has horseshoe-shaped depressions and “tails” oriented in the direction of movement. The surface is covered with a thin matte or black shiny film. There are no obvious deposits, and there are no corrosion products, because the “chelate” (specially introduced into the boiler organic compounds polyamines) has already reacted.
In the presence of oxygen, which rarely happens in a normally operating boiler, the corroded surface is “invigorated”: roughness, islands of metal.
Causes of Corrosion Damage. The mechanism of action of the “chelate” was described earlier (“Industrial and heating boiler houses and mini-CHP”, 1(6)΄ 2011, p. 40).
“Chelate” corrosion occurs when there is an overdose of “chelate,” but it is also possible with a normal dose, since the “chelate” is concentrated in areas where intense evaporation of water occurs: nucleate boiling is replaced by film boiling. In steam separation devices, there are cases of particularly destructive “chelate” corrosion due to high turbulent velocities of water and steam-water mixture.
All of the described corrosion damage can have a synenergetic effect, so that the total damage from the combined action of different corrosion factors can exceed the sum of damage from individual types of corrosion.
As a rule, the action of corrosive agents enhances the unstable thermal regime of the boiler, which causes corrosion fatigue and initiates thermal fatigue corrosion: the number of starts from a cold state is more than 100, the total number of starts is more than 200. Since these types of metal damage occur rarely, cracks, rupture pipes have an appearance identical to metal damage from various types of corrosion.
Usually, to identify the cause of metal destruction, additional metallographic studies are required: radiography, ultrasound, color and magnetic particle flaw detection.
Various researchers have proposed programs for diagnosing types of corrosion damage to boiler steels. The VTI program (A.F. Bogachev and his colleagues) is known - mainly for high-pressure energy boilers, and the developments of the Energochermet association - mainly for low- and medium-pressure energy boilers and waste heat boilers.
Marine site Russia no October 05, 2016 Created: October 05, 2016 Updated: October 05, 2016 Views: 5363Types of corrosion.
Chemical corrosion caused by steam or water, destroys the metal evenly over the entire surface. The rate of such corrosion in modern marine boilers is low. More dangerous is local chemical corrosion caused by aggressive chemical compounds contained in ash deposits (sulfur, vanadium oxides, etc.).
The most common and dangerous is electrochemical corrosion, occurring in aqueous solutions of electrolytes when electric current, caused by potential differences between individual sections of the metal that differ in chemical heterogeneity, temperature or quality of processing.
The role of the electrolyte is played by water (in case of internal corrosion) or condensed water vapor in deposits (in case of external corrosion).
The appearance of such microgalvanic pairs on the surface of the pipes leads to the fact that metal ion atoms pass into water in the form of positively charged ions, and the surface of the pipe in this place acquires a negative charge. If the difference in the potentials of such microgalvanic pairs is insignificant, then a double electric layer is gradually created at the metal-water interface, which slows down the further progress of the process.
However, in most cases, the potentials of individual sections are different, which causes the occurrence of an EMF directed from a higher potential (anode) to a smaller one (cathode).
In this case, metal ion atoms pass from the anode into the water, and excess electrons accumulate at the cathode. As a result, the EMF and, consequently, the intensity of the metal destruction process are sharply reduced.
This phenomenon is called polarization. If the anode potential decreases as a result of the formation of a protective oxide film or an increase in the concentration of metal ions in the anode area, and the cathode potential remains practically unchanged, then the polarization is called anodic.
During cathodic polarization in a solution near the cathode, the concentration of ions and molecules capable of removing excess electrons from the metal surface sharply drops. It follows from this that the main point in the fight against electrochemical corrosion is the creation of conditions where both types of polarization will be maintained.
In practice, this is impossible to achieve, since boiler water always contains depolarizers - substances that disrupt polarization processes.
Depolarizers include O 2 and CO 2 molecules, H + , Cl - and SO - 4 ions, as well as iron and copper oxides. CO 2 , Cl - and SO - 4 dissolved in water inhibit the formation of a dense protective oxide film on the anode and thereby contribute to the intensive occurrence of anodic processes. Hydrogen ions H+ reduce the negative charge of the cathode.
The influence of oxygen on the corrosion rate began to manifest itself in two opposite directions. On the one hand, oxygen increases the rate of the corrosion process, since it is a strong depolarizer of the cathode sites, on the other hand, it has a passivating effect on the surface.
Typically, boiler parts made of steel have a fairly strong initial oxide film, which protects the material from exposure to oxygen until it is destroyed by chemical or mechanical factors.
The rate of heterogeneous reactions (which includes corrosion) is regulated by the intensity of the following processes: supply of reagents (primarily depolarizers) to the surface of the material; destruction of the protective oxide film; removal of reaction products from the site where it occurs.
The intensity of these processes is largely determined by hydrodynamic, mechanical and thermal factors. Therefore, measures to reduce the concentration of aggressive chemical reagents at a high intensity of the other two processes, as experience in operating boilers shows, are usually ineffective.
It follows that the solution to the problem of preventing corrosion damage must be comprehensive, taking into account all the factors influencing the initial causes of destruction of materials.
Electrochemical corrosion
Depending on the place of occurrence and the substances involved in the reactions, the following types of electrochemical corrosion are distinguished:
- oxygen (and its variety - parking),
- sub-sludge (sometimes called “shell”),
- intergranular (alkali brittleness of boiler steels),
- slot and
- sulphurous.
Oxygen corrosion observed in economizers, fittings, feed and downpipe pipes, steam-water collectors and intra-collector devices (boards, pipes, desuperheaters, etc.). Coils of the secondary circuit of double-circuit boilers, recovery boilers and steam air heaters are especially susceptible to oxygen corrosion. Oxygen corrosion occurs during boiler operation and depends on the concentration of oxygen dissolved in the boiler water.
The rate of oxygen corrosion in main boilers is low, which is due to efficient work deaerators and phosphate-nitrate water regime. In auxiliary water-tube boilers it often reaches 0.5 - 1 mm/year, although on average it lies in the range of 0.05 - 0.2 mm/year. The nature of damage to boiler steels is small ulcers.
More dangerous species oxygen corrosion is parking corrosion, occurring during the period of inactivity of the boiler. Due to the specific nature of their work, all ship boilers (and especially auxiliary boilers) are subject to intense docking corrosion. As a rule, stop corrosion does not lead to boiler failures, however, metal that has been corroded during shutdowns, other things being equal, is more intensively destroyed during boiler operation.
The main cause of standstill corrosion is the penetration of oxygen into the water if the boiler is full, or into the moisture film on the metal surface if the boiler is drained. A major role in this is played by chlorides and NaOH contained in water, and water-soluble salt deposits.
If there are chlorides in water, uniform corrosion of the metal intensifies, and if it contains a small amount of alkalis (less than 100 mg/l), then the corrosion is localized. To avoid parking corrosion at a temperature of 20 - 25 ° C, the water should contain up to 200 mg/l NaOH.
External signs of corrosion with the participation of oxygen: small local ulcers (Fig. 1, a), filled with brown corrosion products that form tubercles above the ulcers.
Removing oxygen from feedwater is one of the important measures to reduce oxygen corrosion. Since 1986, the oxygen content in feed water for ships' auxiliary and recovery boilers has been limited to 0.1 mg/l.
However, even with such oxygen content of the feed water, corrosion damage to the boiler elements is observed in operation, which indicates the predominant influence of the processes of destruction of the oxide film and leaching of reaction products from the corrosion sites. Most a clear example, illustrating the influence of these processes on corrosion damage, is the destruction of the coils of recovery boilers with forced circulation.
Rice. 1. Damage due to oxygen corrosion

Corrosion damage with oxygen corrosion, they are usually strictly localized: on inner surface inlet sections (see Fig. 1, a), in the bend area (Fig. 1, b), at the outlet sections and in the coil bend (see Fig. 1, c), as well as in the steam-water collectors of recovery boilers (see. Fig. 1, d). It is in these areas (2 - area of near-wall cavitation) that the hydrodynamic features of the flow create conditions for the destruction of the oxide film and intensive leaching of corrosion products.
Indeed, any deformation of the flow of water and steam-water mixture is accompanied by the appearance cavitation in wall layers expanding flow 2, where the formed and immediately collapsing steam bubbles cause the destruction of the oxide film due to the energy of hydraulic microimpacts.
This is also facilitated by alternating stresses in the film caused by vibration of the coils and fluctuations in temperature and pressure. Increased local turbulization of the flow in these areas causes active leaching of corrosion products.
In the direct outlet sections of the coils, the oxide film is destroyed due to impacts on the surface of water droplets during turbulent pulsations of the flow of the steam-water mixture, the dispersed annular mode of movement of which here becomes dispersed at a flow speed of up to 20-25 m/s.
Under these conditions, even low oxygen content (~ 0.1 mg/l) causes intensive destruction of the metal, which leads to the appearance of fistulas in the inlet sections of the coils of La Mont recovery boilers after 2-4 years of operation, and in other areas - after 6-12 years.
Rice. 2. Corrosion damage to the economizer coils of the recovery boilers KUP1500R of the Indira Gandhi motor ship.

To illustrate the above, let us consider the causes of damage to the economizer coils of two recovery boilers of the KUP1500R type installed on the lighter carrier "Indira Gandhi" (type "Alexey Kosygin"), which entered service in October 1985. Already in February 1987 due to damage The economizers of both boilers were replaced. After 3 years, even in these economizers, damage to the coils appears, located in areas up to 1-1.5 m from the inlet collector. The nature of the damage indicates (Fig. 2, a, b) typical oxygen corrosion followed by fatigue failure (transverse cracks).
However, the nature of fatigue in individual areas is different. The appearance of a crack (and previously, cracking of the oxide film) in the area of the weld (see Fig. 2, a) is a consequence of alternating stresses caused by vibration of the pipe bundle and the design feature of the connection between the coils and the collector (the end of the coil with a diameter of 22x3 is welded to a curved fitting with a diameter of 22x3 22x2).
The destruction of the oxide film and the formation of fatigue cracks on the inner surface of the straight sections of the coils, 700-1000 mm away from the entrance (see Fig. 2, b), are caused by alternating thermal stresses that arise during the commissioning of the boiler, when the hot surface cold water is supplied. In this case, the effect of thermal stresses is enhanced by the fact that the fins of the coils impede the free expansion of the pipe metal, creating additional stresses in the metal.
Sludge corrosion usually observed in main water-tube boilers on the internal surfaces of the screen and steam-generating pipes of the combustion bundles facing the torch. The nature of subsludge corrosion is oval-shaped ulcers with a size along the major axis (parallel to the pipe axis) of up to 30-100 mm.
On the ulcers there is a dense layer of oxides in the form of “shells” 3 (Fig. 3). Slurry corrosion occurs in the presence of solid depolarizers - iron and copper oxides 2, which are deposited on the most heat-stressed sections of pipes in places of active corrosion centers that arise during the destruction of oxide films .
A loose layer of scale and corrosion products forms on top 1. The resulting “shells” of corrosion products are firmly adhered to the base metal and can only be removed mechanically. Under the “shells,” heat transfer deteriorates, which leads to overheating of the metal and the appearance of bulges.
This type of corrosion is not typical for auxiliary boilers, but under high thermal loads and appropriate water treatment conditions, the appearance of sludge corrosion in these boilers cannot be ruled out.
n1.doc
3.4. Corrosion of steam generator elements3.4.1. Corrosion of steam pipesAndsteam generator drums
during their operation
Corrosion damage to the metals of steam generators is caused by one or more factors: excessive heat stress on the heating surface, sluggish water circulation, stagnation of steam, stressed metal, deposition of impurities and other factors that prevent normal washing and cooling of the heating surface.
In the absence of these factors, a normal magnetite film is easily formed and preserved in water with a neutral or moderately alkaline reaction environment that does not contain dissolved oxygen. In the presence of O2, the inlet sections of water economizers, drums and downpipes of circulation circuits may be subject to oxygen corrosion. Low speeds of water movement (in water economizers) have a particularly negative effect, since bubbles of released air are retained in places where the inner surface of the pipes is rough and cause intense local oxygen corrosion. Corrosion of carbon steel in an aqueous environment at high temperatures includes two stages: initial electrochemical and final chemical. According to this corrosion mechanism, divalent iron ions diffuse through the oxide film to the surface of its contact with water, react with hydroxyl or water to form ferrous hydroxide, which then decomposes into magnetite and hydrogen according to the reaction:
| . | (2.4) |
Electrons passing along with iron ions through the oxide film are assimilated by hydrogen ions with the release of H 2. Over time, the thickness of the oxide film increases, and diffusion through it becomes more difficult. As a result, a decrease in the corrosion rate over time is observed.
Nitrite corrosion. If sodium nitrite is present in the feed water, corrosion of the steam generator metal is observed, which has appearance great resemblance with oxygen corrosion. However, unlike it, nitrite corrosion does not affect the inlet sections of the lowering pipes, but the inner surface of the heat-stressed rising pipes and causes the formation of deeper pits with a diameter of up to 15–20 mm.
Nitrites accelerate the cathodic process, and thereby the corrosion of the metal of the steam generator. The course of the process during nitrite corrosion can be described by the following reaction:
| . | (2.5) |
Galvanic corrosion of steam generator metal. The source of galvanic corrosion of steam-generating pipes can be copper entering the steam generators in cases where feed water containing an increased amount of ammonia, oxygen and free carbon dioxide aggressively affects brass and copper pipes regenerative heaters. It should be noted that galvanic corrosion can only be caused by metallic copper deposited on the walls of the steam generator. When maintaining the pH value of the feedwater above 7.6, copper enters the steam generators in the form of oxides or complex compounds, which do not have corrosive properties and are deposited on heating surfaces in the form of sludge. Copper ions present in feed water with a low pH value, entering the steam generator, under alkaline conditions are also precipitated in the form of sludge-like copper oxides. However, under the influence of hydrogen released in steam generators or excess sodium sulfite, copper oxides can be completely reduced to metallic copper, which, deposited on heating surfaces, leads to electrochemical corrosion of the boiler metal.
Sub-sludge (shell) corrosion. Sludge corrosion occurs in stagnant zones of the circulation circuit of a steam generator under a layer of sludge consisting of metal corrosion products and phosphate treatment of boiler water. If these deposits are concentrated in heated areas, then intense evaporation occurs underneath them, increasing the salinity and alkalinity of the boiler water to dangerous values.
Sludge corrosion spreads in the form of large pits with a diameter of up to 50–60 mm on the inside of the steam-generating pipes facing the furnace torch. Within the ulcers, a relatively uniform decrease in the thickness of the pipe wall is observed, often leading to the formation of fistulas. On the ulcers a dense layer of iron oxides in the form of shells is found. The described destruction of metal is called “shell” corrosion in the literature. Sludge corrosion, caused by oxides of ferric iron and divalent copper, is an example of combined metal destruction; The first stage of this process is purely electrochemical, and the second is chemical, caused by the action of water and water vapor on overheated areas of the metal located under the layer of sludge. The most effective means of combating “shell” corrosion of steam generators is to prevent the occurrence of corrosion of the feed water path and the removal of iron and copper oxides from it with the feed water.
Alkali corrosion. The stratification of the steam-water mixture, which occurs in horizontal or slightly inclined steam-generating pipes, is known to be accompanied by the formation of steam bags, overheating of the metal and deep evaporation of the boiler water film. The highly concentrated film formed during the evaporation of boiler water contains a significant amount of alkali in the solution. Caustic soda, present in boiler water in small concentrations, protects the metal from corrosion, but it becomes a very dangerous corrosion factor if conditions are created on any areas of the surface of the steam generator for deep evaporation of boiler water with the formation of an increased concentration of NaOH.
The concentration of caustic soda in the evaporated film of boiler water depends on:
A) on the degree of overheating of the wall of the steam-generating pipe compared to the boiling point at a given pressure in the steam generator, i.e. quantities?t s;
B) the ratios of the concentration of caustic soda and sodium salts contained in circulating water, which have the ability to greatly increase the boiling point of water at a given pressure.
If the concentration of chlorides in the boiler water significantly exceeds the concentration of NaOH in an equivalent ratio, then before the latter reaches dangerous values in the evaporating film, the content of chlorides in it increases so much that the boiling point of the solution exceeds the temperature of the superheated pipe wall, and further evaporation of water stops. If the boiler water contains predominantly caustic soda, then at ?t s = 7 °C the concentration of NaOH in the film of concentrated water is 10%, and at
?t s = 30 °C reaches 35%. Meanwhile, it has been established experimentally that already 5–10% solutions of caustic soda at boiler water temperatures above 200 °C are capable of intensively corroding the metal of heated areas and welds with the formation of loose magnetic ferrous oxide and the simultaneous release of hydrogen. Alkaline corrosion is selective, moving deeper into the metal mainly along pearlite grains and forming a network of intercrystalline cracks. A concentrated solution of caustic soda is also capable of dissolving the protective layer of iron oxides at high temperatures to form sodium ferrite NaFeO 2, which hydrolyzes to form an alkali:
| | (2.6) |
| | (2.7) |
Due to the fact that alkali is not consumed in this circular process, the possibility of a continuous corrosion process is created. The higher the temperature of the boiler water and the concentration of caustic soda, the more intense the process of alkaline corrosion occurs. It has been established that concentrated solutions of caustic soda not only destroy the protective magnetite film, but also inhibit its recovery after damage.
The source of alkaline corrosion of steam generators can also be sludge deposits, which contribute to deep evaporation of boiler water with the formation of a highly concentrated, corrosive alkali solution. Reducing the relative proportion of alkali in the total salt content of boiler water and creating a predominant content of salts such as chlorides in the latter can dramatically reduce alkaline corrosion of boiler metal. Elimination of alkaline corrosion is also achieved by ensuring the cleanliness of the heating surface and intensive circulation in all areas of the steam generator, which prevents deep evaporation of water.
Intergranular corrosion. Intergranular corrosion occurs as a result of the interaction of boiler metal with alkaline boiler water. Feature intercrystalline cracks are that they occur in places of greatest stress in the metal. Mechanical stresses are composed of internal stresses arising during the manufacture and installation of drum-type steam generators, as well as additional stresses arising during operation. The formation of intergranular ring cracks on pipes is promoted by additional static mechanical stresses. They occur in pipe circuits and in steam generator drums with insufficient compensation for temperature expansion, as well as due to uneven heating or cooling of individual parts of the drum or collector body.
Intercrystalline corrosion occurs with some acceleration: in the initial period, the destruction of the metal occurs very slowly and without deformation, and then over time its speed increases sharply and can take on catastrophic proportions. Intergranular corrosion of boiler metal should be considered primarily as a special case of electrochemical corrosion occurring along the grain boundaries of stressed metal in contact with an alkaline concentrate of boiler water. The appearance of corrosive microgalvanic elements is caused by the difference in potentials between the bodies of crystallites that act as cathodes. The role of anodes is played by the collapsing grain faces, the potential of which, due to mechanical stress metal in this place is greatly reduced.
Along with electrochemical processes, atomic hydrogen, a discharge product, plays a significant role in the development of intergranular corrosion
H + -ions on the cathode of corrosion elements; easily diffusing into the thickness of steel, it destroys carbides and creates large internal stresses in the metal of the boiler due to the appearance of methane in it, which leads to the formation of thin intergranular cracks (hydrogen cracking). In addition, during the reaction of hydrogen with steel inclusions, various gaseous products are formed, which in turn causes additional tensile forces and promotes loosening of the structure, deepening, expansion and branching of cracks.
The main way to prevent hydrogen corrosion of the boiler metal is to eliminate any corrosion processes leading to the formation of atomic hydrogen. This is achieved by weakening the deposit of iron and copper oxides in the steam generator, chemical cleaning of boilers, improving water circulation and reducing local increased thermal loads of the heating surface.
It has been established that intergranular corrosion of boiler metal in the joints of steam generator elements occurs only in the simultaneous presence of local tensile stresses close to or exceeding the yield strength, and when the concentration of NaOH in the boiler water, accumulating in leaks in the joints of boiler elements, exceeds 5–6%. For the development of intergranular fractures of boiler metal, it is not essential absolute value alkalinity, and the proportion of caustic soda in the total salt composition of boiler water. It has been established experimentally that if this proportion, i.e., the relative concentration of caustic soda in boiler water, is less than 10–15% of the amount of mineral soluble substances, then such water, as a rule, is not aggressive.
Steam-water corrosion. In places with defective circulation, where steam stagnates and is not immediately discharged into the drum, the walls of the pipes under the steam bags are subject to severe local overheating. This leads to chemical corrosion of the metal of the steam-generating pipes, overheated to 450 °C and above, under the influence of highly superheated steam. The process of corrosion of carbon steel in highly superheated water vapor (at a temperature of 450 - 470 ° C) comes down to the formation of Fe 3 O 4 and hydrogen gas:
| | (2.8.) |
It follows that the criterion for the intensity of steam-water corrosion of the boiler metal is an increase in the content of free hydrogen in saturated steam. Steam-water corrosion of steam-generating pipes is observed, as a rule, in zones of sharp fluctuations in wall temperature, where heat changes occur, causing the destruction of the protective oxide film. This creates the possibility of direct contact of the superheated metal of the pipe with water or water vapor and chemical interaction between them.
Corrosion fatigue. In the drums of steam generators and boiler pipes, if the metal is exposed simultaneously to a corrosive environment by thermal stresses of variable sign and magnitude, corrosion fatigue cracks deeply penetrating into the steel appear, which can be transgranular, intercrystalline, or mixed in nature. As a rule, cracking of boiler metal is preceded by the destruction of the protective oxide film, which leads to significant electrochemical heterogeneity and, as a consequence, to the development of local corrosion.
In steam generator drums, corrosion fatigue cracks occur during alternating heating and cooling of the metal in small areas at the junction of pipelines (feed water, periodic purging, injection of phosphate solution) and water-indicating columns with the drum body. In all these connections, the drum metal is cooled if the temperature of the feed water flowing through the pipe is less than the saturation temperature at the pressure in the steam generator. Local cooling of the drum walls followed by heating them with hot boiler water (at times of power failure) is always associated with the appearance of high internal stresses in the metal.
Corrosion cracking of steel sharply increases under conditions of alternate wetting and drying of the surface, as well as in cases where the movement of the steam-water mixture through the pipe has a pulsating nature, i.e., the speed of movement of the steam-water mixture and its steam content often and sharply change, as well as during a kind of stratification steam-water mixture into separate “plugs” of steam and water, following each other.
3.4.2. Superheater corrosion
The rate of steam-water corrosion is determined primarily by the temperature of the steam and the composition of the metal in contact with it. The magnitude of heat exchange and temperature fluctuations during operation of the superheater are also of significant importance in its development, as a result of which destruction of protective oxide films can be observed. In an environment of superheated steam with a temperature greater
575 °C FeO (wustite) is formed on the steel surface as a result of steam-water corrosion:
It has been established that pipes made of ordinary low-carbon steel, when exposed to highly superheated steam for a long time, are uniformly destroyed with simultaneous degeneration of the metal structure and the formation of a dense layer of scale. In ultra-high and supercritical pressure steam generators at a steam superheat temperature of 550 °C and above, the most thermally stressed elements of the superheater (output sections) are usually made of heat-resistant austenitic stainless steels (chromium-nickel, chromium-molybdenum, etc.). These steels are subject to cracking under the combined action of tensile stresses and a corrosive environment. Most operational damage to steam superheaters, characterized by corrosion cracking of elements made of austenitic steels, is caused by the presence of chlorides and caustic soda in the steam. The fight against corrosion cracking of parts made of austenitic steels is carried out mainly by maintaining a safe water regime in steam generators.
3.4.3. Standstill corrosion of steam generators
When steam generators or other steam power equipment are idle in cold or hot reserve or during repairs, so-called standing corrosion develops on the metal surface under the influence of atmospheric oxygen or moisture. For this reason, equipment downtime without proper corrosion protection measures often results in serious damage, especially in steam generators. Superheaters and steam-generating pipes in the transition zones of direct-flow steam generators suffer greatly from standstill corrosion. One of the reasons for standstill corrosion of the internal surface of steam generators is their filling with oxygen-saturated water during downtime. In this case, the metal at the water-air interface is especially susceptible to corrosion. If a steam generator left for repairs is completely drained, then a film of moisture always remains on its inner surface with the simultaneous access of oxygen, which, easily diffusing through this film, causes active electrochemical corrosion of the metal. Thin film moisture is retained for quite a long time, since the atmosphere inside the steam generator is saturated with water vapor, especially if steam enters it through leaks in the fittings of parallel operating steam generators. If the water filling the reserve steam generator contains chlorides, this leads to an increase in the rate of uniform corrosion of the metal, and if it contains a small amount of alkali (less than 100 mg/dm 3 NaOH) and oxygen, this contributes to the development of pitting corrosion.
The development of standstill corrosion is also facilitated by sludge accumulating in the steam generator, which usually retains moisture. For this reason, significant corrosion pits are often found in drums along the lower generatrix at their ends, i.e., in areas of greatest accumulation of sludge. Particularly susceptible to corrosion are areas of the internal surface of steam generators that are covered with water-soluble salt deposits, such as superheater coils and the transition zone in once-through steam generators. During steam generator downtime, these deposits absorb atmospheric moisture and spread to form a highly concentrated solution of sodium salts on the metal surface, which has high electrical conductivity. With free access of air, the corrosion process under salt deposits proceeds very intensively. It is very significant that standstill corrosion intensifies the process of corrosion of the boiler metal during operation of the steam generator. This circumstance should be considered the main danger of parking corrosion. The resulting rust, consisting of high-valence iron oxides Fe(OH) 3, during operation of the steam generator plays the role of a depolarizer of corrosive micro- and macrogalvanic couples, which leads to intensified metal corrosion during operation of the unit. Ultimately, the accumulation of rust on the metal surface of the boiler leads to sludge corrosion. In addition, during subsequent downtime of the unit, the restored rust again acquires the ability to cause corrosion due to its absorption of oxygen from the air. These processes are repeated cyclically during alternating downtime and operation of steam generators.
Various preservation methods are used to protect steam generators from static corrosion during periods of inactivity in reserve and for repairs.
3.5. Corrosion steam turbines
During operation, the metal of the turbine flow path may be subject to corrosion in the steam condensation zone, especially if it contains carbonic acid, cracking due to the presence of corrosive agents in the steam and standstill corrosion when the turbines are in reserve or under repair. The flow part of the turbine is especially susceptible to standstill corrosion if there are salt deposits in it. The saline solution formed during turbine downtime accelerates the development of corrosion. This implies the need for thorough cleaning of turbine blade apparatus from deposits before long downtime her.
Corrosion during idle periods is usually relatively uniform; under unfavorable conditions, it manifests itself in the form of numerous pits evenly distributed over the metal surface. The place where it flows are those stages where moisture condenses, aggressively affecting the steel parts of the turbine flow path.
The source of moisture is primarily the condensation of steam filling the turbine after it stops. The condensate partially remains on the blades and diaphragms, and partially drains and accumulates in the turbine housing, since it is not discharged through drains. The amount of moisture inside the turbine may increase due to steam leakage from the extraction and backpressure steam lines. The internal parts of the turbine are always cooler than the air entering the turbine. The relative humidity of the air in the machine room is very high, so a slight cooling of the air is enough for the dew point to reach and moisture to form on metal parts.
To eliminate standstill corrosion of steam turbines, it is necessary to exclude the possibility of steam entering the turbines while they are in reserve, both from the side of the superheated steam steam line and from the side of the extraction line, drainage lines, etc. To maintain the surface of the blades, disks and rotor dry This method involves periodically blowing the internal cavity of the reserve turbine with a stream of hot air (t = 80 h 100 °C), supplied by a small auxiliary fan through a heater (electric or steam).
3.6. Corrosion of turbine condensers
Under operating conditions of steam power plants, cases of corrosion damage to brass condenser pipes are often observed, both on the inside, washed by cooling water, and on the outside. The internal surfaces of condenser pipes, cooled by highly mineralized, salty lake waters containing large amounts of chlorides, or by circulating circulating waters with increased mineralization and contaminated suspended particles, intensively corrode.
A characteristic feature of brass is how construction material is its tendency to corrosion under the combined action of increased mechanical stress and an environment with even moderate aggressive properties. Corrosion damage occurs in brass tube condensers in the form of general dezincification, plug dezincification, corrosion cracking, impact corrosion and corrosion fatigue. The occurrence of the noted forms of corrosion of brass is decisively influenced by the composition of the alloy, the manufacturing technology of condenser tubes and the nature of the contacted medium. Due to dezincification, the destruction of the surface of brass pipes can be of a continuous layer nature or belong to the so-called plug type, which is the most dangerous. Cork dezincification is characterized by pits that go deep into the metal and are filled with loose copper. The presence of through fistulas makes it necessary to replace the pipe in order to avoid the suction of cooling raw water into the condensate.
Conducted studies, as well as long-term observations of the condition of the surface of condenser tubes in operating capacitors, have shown that the additional introduction of small amounts of arsenic into brass significantly reduces the tendency of brasses to dezincification. Composite brasses, additionally alloyed with tin or aluminum, also have increased corrosion resistance due to the ability of these alloys to quickly restore protective films when they are mechanically destroyed. Due to the use of metals occupying various places in the potential series and electrically connected, macroelements appear in the capacitor. The presence of an alternating temperature field creates the possibility of developing corrosive and dangerous EMF of thermoelectric origin. Stray currents arising when grounding nearby direct current, can also cause severe corrosion of capacitors.
Corrosion damage to condenser tubes from condensing steam is most often associated with the presence of ammonia in it. The latter, being a good complexing agent with respect to copper and zinc ions, creates favorable conditions for dezincification of brass. In addition, ammonia causes corrosion cracking of brass condenser tubes in the presence of internal or external tensile stresses in the alloy, which gradually widen the cracks as the corrosion process develops. It has been established that in the absence of oxygen and other oxidizing agents, ammonia solutions cannot have an aggressive effect on copper and its alloys; therefore, there is no need to worry about ammonia corrosion of brass pipes when the ammonia concentration in the condensate is up to 10 mg/dm 3 and lack of oxygen. In the presence of even a small amount of oxygen, ammonia destroys brass and other copper alloys at a concentration of 2–3 mg/dm3 .
Corrosion from the steam side may primarily affect the brass pipes of vapor coolers, ejectors and air suction chambers of turbine condensers, where conditions are created that favor the entry of air and the occurrence of local increased concentrations of ammonia in partially condensed steam.
To prevent corrosion of condenser tubes on the water side, each specific case when choosing metal or alloys suitable for the manufacture of these pipes, take into account their corrosion resistance for a given composition of cooling water. Particularly serious attention to the selection of corrosion-resistant materials for the manufacture of condenser pipes should be given in cases where the condensers are cooled by running highly mineralized water, as well as in conditions of replenishment of losses of cooling water in the circulating water supply systems of thermal power plants, fresh waters with high mineralization, or contaminated with corrosive industrial and household waste.
3.7. Corrosion of make-up and network equipment
3.7.1. Corrosion of pipelines and hot water boilers
A number of power plants use river and tap water with a low pH value and low hardness to feed heating networks. Additional treatment of river water at a waterworks usually leads to a decrease in pH, a decrease in alkalinity and an increase in the content of aggressive carbon dioxide. The appearance of aggressive carbon dioxide is also possible in acidification schemes used for large heat supply systems with direct hot water supply (2000–3000 t/h). Softening water according to the Na cationization scheme increases its aggressiveness due to the removal of natural corrosion inhibitors - hardness salts.
With poorly established water deaeration and possible increases in oxygen and carbon dioxide concentrations due to the lack of additional protective measures in heat supply systems, pipelines, heat exchangers, storage tanks and other equipment are susceptible to internal corrosion.
It is known that an increase in temperature promotes the development of corrosion processes that occur both with the absorption of oxygen and with the release of hydrogen. With an increase in temperature above 40 °C, oxygen and carbon dioxide forms of corrosion increase sharply.
A special type of sludge corrosion occurs under conditions of low residual oxygen content (if PTE standards are met) and when the amount of iron oxides exceeds 400 μg/dm 3 (in terms of Fe). This type of corrosion, previously known in the practice of operating steam boilers, was discovered under conditions of relatively weak heating and the absence of thermal loads. In this case, loose corrosion products, consisting mainly of hydrated ferric oxides, are active depolarizers of the cathodic process.
When operating heating equipment, crevice corrosion is often observed, i.e., selective, intense corrosion destruction of metal in a crevice (gap). A feature of the processes occurring in narrow gaps is a reduced oxygen concentration compared to the concentration in the solution volume and a slow removal of corrosion reaction products. As a result of the accumulation of the latter and their hydrolysis, a decrease in the pH of the solution in the gap is possible.
When a heating network with an open water supply is constantly fed with deaerated water, the possibility of the formation of through fistulas on pipelines is completely eliminated only under normal hydraulic conditions, when excess pressure above atmospheric pressure is constantly maintained at all points of the heating supply system.
The causes of pitting corrosion of hot water boiler pipes and other equipment are as follows: poor deaeration of make-up water; low pH value due to the presence of aggressive carbon dioxide (up to 10–15 mg/dm 3); accumulation of oxygen corrosion products of iron (Fe 2 O 3) on heat transfer surfaces. An increased content of iron oxides in network water contributes to the contamination of boiler heating surfaces with iron oxide deposits.
A number of researchers recognize the important role in the occurrence of sub-sludge corrosion of the process of rusting of pipes of water heating boilers during their downtime, when proper measures have not been taken to prevent standstill corrosion. Foci of corrosion that arise under the influence of atmospheric air on the wet surfaces of boilers continue to function during operation of the boilers.
3.7.2. Corrosion of heat exchanger tubes
The corrosion behavior of copper alloys depends significantly on temperature and is determined by the presence of oxygen in water.
In table Table 3.1 shows the rate of transition of corrosion products of copper-nickel alloys and brass into water at high (200 μg/dm 3) and low
(3 µg/dm 3) oxygen content. This rate is approximately proportional to the corresponding corrosion rate. It increases significantly with increasing oxygen concentration and salt content of water.
In acidification schemes, the water after the decarbonizer often contains up to 5 mg/dm 3 of carbon dioxide, while the service life of the tubular bundle of L-68 brass heaters is 9–10 months.
Table 3.1
The rate of transition of corrosion products into water from the surface
copper-nickel alloys and brass in a neutral environment, 10 -4 g/(m 2 h)
| Material | O 2 content, µg/dm 3 | Temperature, °C |
||||
| 38 | 66 | 93 | 121 | 149 |
||
| MN 70-30 MN 90-10 LO-70-1 | 3 | - | 3,8 | 4,3 | 3,2 | 4,5 |
Hard and soft deposits formed on the surface have a significant influence on the corrosion destruction of tubes. The nature of these deposits is important. If deposits are capable of filtering water and at the same time can retain copper-containing corrosion products on the surface of the tubes, the local process of destruction of the tubes intensifies. Deposits with a porous structure (hard scale deposits, organic) have a particularly adverse effect on the course of corrosion processes. With an increase in water pH, the permeability of carbonate films increases, and with an increase in its hardness, it sharply decreases. This explains that in circuits with starved regeneration of filters, corrosion processes occur less intensely than in Na-cationization circuits. The service life of the tubes is also reduced by contamination of their surface with corrosion products and other deposits, leading to the formation of ulcers under the deposits. With timely removal of contaminants, local corrosion of tubes can be significantly reduced. Accelerated failure of heaters with brass tubes is observed with increased salt content of water - more than 300 mg/dm 3, and chloride concentrations - more than 20 mg/dm 3.
Average term The service life of heat exchanger tubes (3–4 years) can be increased if they are made from corrosion-resistant materials. Stainless steel tubes 1Х18Н9Т, installed in the make-up duct at a number of thermal power plants with low-mineralized water, have been in operation for more than 7 years without signs of damage. However, at present it is difficult to count on the widespread use of stainless steels due to their high scarcity. It should also be kept in mind that these steels are susceptible to pitting corrosion at elevated temperatures, salinity, chloride concentrations, and sediment contamination.
When the salt content of make-up and supply water is higher than 200 mg/dm 3 and chlorine ions is higher than 10 mg/dm 3, it is necessary to limit the use of L-68 brass, especially in the make-up tract to the deaerator, regardless of the water preparation scheme. When using softened make-up water containing significant amounts of aggressive carbon dioxide (over 1 mg/dm 3), the flow rate in devices with a brass pipe system must exceed 1.2 m/s.
MNZh-5-1 alloy should be used when the heating network make-up water temperature is above 60 °C.
Table 3.2
Metal tubes of heat exchangers depending on
From the heating network make-up water treatment scheme
| Makeup water treatment scheme | Metal of heat exchanger tubes in the path to the deaerator | Metal tubes of network heat exchangers |
| Liming | L-68, LA-77-2 | L-68 |
| Na-cationization | LA-77-2, MNZH-5-1 | L-68 |
| H-cationization with starvation filter regeneration | LA-77-2, MNZH-5-1 | L-68 |
| Acidification | LA-77-2, MNZH-5-1 | L-68 |
| Soft water without treatment W o = 0.5 h 0.6 mmol/dm 3, Sh o = 0.2 h 0.5 mmol/dm 3, pH = 6.5 h 7.5 | LA-77-2, MNZH-5-1 | L-68 |
3.7.3. Assessment of the corrosion state of existingsystems
hotwater supply and reasonscorrosion
Hot water supply systems compared to other engineering structures (heating, cold water supply and sewerage systems) are the least reliable and durable. If the established and actual service life of buildings is estimated at 50–100 years, and heating, cold water supply and sewerage systems are estimated at 20–25 years, then for hot water supply systems with a closed heat supply scheme and communications made of uncoated steel pipes, the actual service life does not exceed 10 years, and in some cases 2–3 years.
Hot water supply pipelines without protective coatings are susceptible to internal corrosion and significant contamination with its products. This leads to a decrease in communications capacity, an increase in hydraulic losses and disruptions in the supply of hot water, especially in upper floors buildings with insufficient pressure from the city water supply. IN large systems hot water supply from central heating points, the overgrowing of pipelines with corrosion products disrupts the regulation of branched systems and leads to interruptions in the supply of hot water. Due to intense corrosion, especially of external hot water supply networks from central heating stations, the volume of current and major repairs is increasing. The latter are associated with frequent relocations of internal (in houses) and external communications, disruption of the improvement of urban areas within neighborhoods, and long-term interruption of hot water supply to a large number of consumers when the head sections of hot water supply pipelines fail.
Corrosion damage to hot water supply pipelines from central heating stations in the event of joint laying with heating distribution networks lead to flooding of the latter with hot water and their intense external corrosion. At the same time, great difficulties arise in detecting accident sites, it is necessary to carry out a large amount of excavation work and deteriorate the amenities of residential areas.
With minor differences in capital investments for the construction of hot, cold water supply and heating systems operating costs associated with frequent relocation and repair of hot water supply communications are disproportionately higher.
Corrosion of hot water supply systems and protection against it are of particular importance due to the scale of housing construction in Russia. The tendency to consolidate the capacity of individual installations leads to a branching network of hot water supply pipelines, which are usually made from ordinary steel pipes without protective coatings. The ever-increasing shortage of drinking-quality water necessitates the use of new sources of water with high corrosive activity.
One of the main reasons affecting the condition of hot water supply systems is the high corrosiveness of heated tap water. According to VTI research, the corrosive activity of water, regardless of the source of water supply (surface or underground), is characterized by three main indicators: the index of equilibrium water saturation with calcium carbonate, the content of dissolved oxygen and the total concentration of chlorides and sulfates. Previously, the domestic literature did not provide a classification of heated tap water by corrosive activity depending on the parameters of the source water.
In the absence of conditions for the formation of protective carbonate films on the metal (j
Observational data from existing hot water supply systems indicate a significant influence of chlorides and sulfates in tap water on pipeline corrosion. Thus, waters, even with a positive saturation index, but containing chlorides and sulfates in concentrations above 50 mg/dm 3, are corrosive, which is due to a violation of the continuity of carbonate films and a decrease in their protective action under the influence of chlorides and sulfates. When the protective films are destroyed, the chlorides and sulfates present in the water increase the corrosion of steel under the influence of oxygen.
Based on the corrosion scale adopted in thermal power engineering and experimental data from VTI, a conditional corrosion classification of tap water at a design temperature of 60 °C is proposed based on the corrosion rate of steel pipes in heated drinking water (Table 3.3). 
Rice. 3.2. Dependence of the depth index P of corrosion of steel pipes in heated tap water (60 °C) on the calculated saturation index J:
1, 2, 3 – surface source  ; 4 – underground source
; 4 – underground source  ; 5 – surface source
; 5 – surface source 
In Fig. 3.2. experimental data on the corrosion rate in samples of steel pipes at different qualities of tap water are presented. The graph shows a certain pattern of decrease in the depth corrosion index (depth permeability) with a change in the calculated water saturation index (with a content of chlorides and sulfates up to 50 mg/dm 3). With negative values of the saturation index, deep permeability corresponds to emergency and severe corrosion (points 1 and 2) ;
for river water with a positive saturation index (point 3) there is acceptable corrosion, and for artesian water (point 4) there is weak corrosion. Noteworthy is the fact that for artesian and river water with a positive saturation index and a content of chlorides and sulfates less than 50 mg/dm 3, the differences in the depth of corrosion permeability are relatively small. This means that in waters prone to the formation of an oxide-carbonate film on pipe walls (j > 0), the presence of dissolved oxygen (high in surface water and insignificant in underground water) does not have a noticeable effect on the change in deep corrosion permeability. At the same time, test data (point 5) indicate a significant increase in the intensity of steel corrosion in water with a high concentration of chlorides and sulfates (in total about 200 mg/dm 3), despite the positive saturation index (j = 0.5). Corrosion permeability in this case corresponds to permeability in water having a saturation index j = – 0.4. In accordance with the classification of waters according to corrosive activity, water with a positive saturation index and a high content of chlorides and sulfates is classified as corrosive.
Table 3.3
Classification of water by corrosiveness
| J at 60 °C | Concentration in cold water, mg/dm3 | Corrosion characteristics of heated water (at 60 °C) |
|
| dissolved oxygen O 2 | chlorides and sulfates (in total)  |
||
 | Any | Any | Highly corrosive |
 | Any | >50 | Highly corrosive |
| | Any |  | Corrosive |
 | Any | >50 | Slightly corrosive |
| | >5 | | Slightly corrosive |
| |  | | Non-corrosive |
The classification developed by VTI (Table 3.3) quite fully reflects the influence of water quality on its corrosion properties, which is confirmed by data on the actual corrosion state of hot water supply systems.
Analysis of the main indicators of tap water in a number of cities allows us to classify the majority of water as highly corrosive and corrosive, and only a small part as slightly corrosive and non-corrosive. A large proportion of sources are characterized by increased concentrations of chlorides and sulfates (more than 50 mg/dm 3), and there are examples when these concentrations in total reach 400–450 mg/dm 3. Such a significant content of chlorides and sulfates in tap water determines their high corrosive activity.
When assessing corrosion activity surface waters it is necessary to take into account the variability of their composition throughout the year. For a more reliable assessment, you should use data from not just a single, but as many as possible water analyzes performed in different seasons over the last one or two years.
For artesian springs, water quality indicators are usually very stable throughout the year. As a rule, groundwater is characterized by increased mineralization, a positive saturation index for calcium carbonate and a high total content of chlorides and sulfates. The latter leads to the fact that hot water supply systems in some cities, receiving water from artesian wells, are also subject to severe corrosion.
When there are several sources of drinking water in one city, the intensity and scale of corrosion damage to hot water supply systems can be different. Thus, in Kyiv there are three sources of water supply:
R. Dnepr, r. Desna and artesian wells. Hot water supply systems in areas of the city supplied with corrosive Dnieper water are most susceptible to corrosion; to a lesser extent - systems operated with slightly corrosive Desnyansk water, and to an even lesser extent - with artesian water. The presence of areas in the city with different corrosive characteristics of tap water greatly complicates the organization of anti-corrosion measures both at the design stage and during the operation of hot water supply systems.
To assess the corrosion state of hot water supply systems, surveys were carried out in a number of cities. Experimental studies of the corrosion rate of pipes using tubular and plate samples were carried out in areas of new housing construction in the cities of Moscow, St. Petersburg, etc. The survey results showed that the condition of pipelines is directly dependent on the corrosive activity of tap water.
A significant influence on the extent of corrosion damage in the hot water supply system is exerted by the high centralization of water heating installations at central heating points or heat distribution stations (DHS). Initially, the widespread construction of central heating stations in Russia was due to a number of reasons: the lack of basements in new residential buildings suitable for placing hot water supply equipment; inadmissibility of installing conventional (non-silent) circulation pumps in individual heating points; the expected reduction in service personnel as a result of the replacement of relatively small heaters installed in individual heating points with large ones; the need to increase the level of operation of central heating stations by automating them and improving service; the possibility of constructing large installations for anti-corrosion treatment of water for hot water supply systems.
However, as experience in operating central heating stations and hot water supply systems from them has shown, the number of service personnel has not been reduced due to the need to perform a large amount of work during routine and major repairs of hot water supply systems. Centralized anti-corrosion treatment of water at central heating stations has not become widespread due to the complexity of the installations, high initial and operating costs and the lack of standard equipment (vacuum deaeration).
In conditions where predominantly steel pipes without protective coatings are used for hot water supply systems, with the high corrosive activity of tap water and the absence of anti-corrosion water treatment at the central heating station, further construction of the central heating station alone is apparently inappropriate. Construction in recent years of houses of new series with basements and production of silent centrifugal pumps will contribute in many cases to the transition to the design of individual heating points (IHP) and increasing the reliability of hot water supply.
3.8. Conservation of thermal power equipment
and heating networks
3.8.1. General position
Preservation of equipment is protection against so-called parking corrosion.
Preservation of boilers and turbine units to prevent corrosion of the metal of internal surfaces is carried out during routine shutdowns and withdrawal to reserve for a definite and indefinite period: withdrawal - for current, medium, major repairs; emergency shutdowns, for long-term reserve or repair, for reconstruction for a period exceeding 6 months.
Based production instructions at each power plant and boiler house, a technical solution for organizing the conservation of specific equipment must be developed and approved, defining methods of conservation for various types of shutdowns and the duration of downtime of the technological scheme and auxiliary equipment.
When developing a technological conservation scheme, it is advisable to use as much as possible standard settings correctional treatment of feed and boiler water, installations for chemical cleaning of equipment, tank facilities of the power plant.
The technological conservation scheme should be as stationary as possible and reliably disconnected from the operating sections of the thermal circuit.
It is necessary to provide for the neutralization or neutralization of waste water, as well as the possibility of reusing preservative solutions.
In accordance with accepted technical solution instructions for the preservation of equipment are drawn up and approved with instructions on preparatory operations, preservation and re-preservation technologies, as well as safety measures during conservation.
When preparing and carrying out conservation and re-preservation work, it is necessary to comply with the requirements of the Safety Rules for the operation of thermal mechanical equipment of power plants and heating networks. Also, if necessary, should be taken additional measures safety related to the properties of the chemical reagents used.
Neutralization and purification of spent preservative solutions of chemical reagents must be carried out in accordance with directive documents.
3.8.2. Methods for preserving drum boilers
1. “Dry” shutdown of the boiler.
Dry shutdown is used for boilers of any pressure if they do not have rolling connections between pipes and drums.
A dry shutdown is carried out during a planned shutdown for reserve or repair for up to 30 days, as well as during an emergency shutdown.
The dry shutdown technique is as follows.
After stopping the boiler during its natural cooling or cooling, drainage begins at a pressure of 0.8 - 1.0 MPa. The intermediate superheater is steamed to a condenser. After drainage, close all valves and valves of the steam-water circuit of the boiler.
Draining the boiler at a pressure of 0.8 - 1.0 MPa allows, after emptying it, to maintain the temperature of the metal in the boiler above the saturation temperature at atmospheric pressure due to heat accumulated by metal, lining and insulation. In this case, the internal surfaces of the drum, collectors and pipes are dried.
2. Maintaining excess pressure in the boiler.
Maintaining a pressure above atmospheric pressure in the boiler prevents oxygen and air from entering it. Excessive pressure is maintained by flowing deaerated water through the boiler. Preservation while maintaining excess pressure is used for boilers of any type and pressure. This method is carried out when the boiler is put into reserve or repairs not related to work on heating surfaces for up to 10 days. On boilers with rolling connections between pipes and drums, it is allowed to use excess pressure for up to 30 days.
3. In addition to the above preservation methods, the following are used on drum boilers:
Hydrazine treatment of heating surfaces at boiler operating parameters;
Hydrazine treatment at reduced steam parameters;
Hydrazine “boil-down” of boiler heating surfaces;
Trilon treatment of boiler heating surfaces;
Phosphate-ammonia “dilution”;
Filling the heating surfaces of the boiler with protective alkaline solutions;
Filling the heating surfaces of the boiler with nitrogen;
Preservation of the boiler with a contact inhibitor.
3.8.3. Methods for preserving once-through boilers
1. “Dry” shutdown of the boiler.
Dry stop applies to all once-through boilers regardless of the adopted water chemistry regime. It is carried out during any planned and emergency shutdowns for up to 30 days. Steam from the boiler is partially released into the condenser so that within 20–30 minutes the pressure in the boiler drops to
30–40 kgf/cm 2 (3–4 MPa). Open the drains of the inlet manifolds and the water economizer. When the pressure drops to zero, the boiler is evaporated to the condenser. The vacuum is maintained for at least 15 minutes.
2. Hydrazine and oxygen treatment of heating surfaces at boiler operating parameters.
Hydrazine and oxygen treatment is carried out in combination with a dry shutdown. The technique for carrying out hydrazine treatment of a once-through boiler is the same as for a drum boiler.
3. Filling the heating surfaces of the boiler with nitrogen.
The boiler is filled with nitrogen at excess pressure in the heating surfaces. Nitrogen preservation is used on boilers of any pressure at power plants that have nitrogen from their own installations!
4. Preservation of the boiler with a contact inhibitor.
Boiler preservation with a contact inhibitor is used for all types of boilers, regardless of the water chemistry regime used, and is carried out when the boiler is put into reserve or repaired for a period of 1 month to 2 years.
3.8.4. Methods for preserving hot water boilers
1. Preservation with calcium hydroxide solution.
The protective film remains for 2–3 months after the boiler is emptied of solution after 3–4 or more weeks of contact. Calcium hydroxide is used for the preservation of hot water boilers of any type at power plants, boiler houses with water treatment plants with lime facilities. The method is based on the highly effective inhibitory abilities of a solution of calcium hydroxide Ca(OH) 2. The protective concentration of calcium hydroxide is 0.7 g/dm3 and higher. Upon contact with metal, its stable protective film is formed within 3–4 weeks.
2. Preservation with sodium silicate solution.
Sodium silicate is used for the preservation of hot water boilers of any type when the boiler is put into reserve for a period of up to 6 months or when the boiler is taken out for repairs for a period of up to 2 months.
Sodium silicate (liquid sodium glass) forms a strong protective film on the metal surface in the form of the Fe 3 O 4 ·FeSiO 3 compound. This film shields the metal from the effects of corrosive agents (CO 2 and O 2). When implementing this method, the hot water boiler is completely filled with a sodium silicate solution with a SiO 2 concentration in the preservative solution of at least 1.5 g/dm 3 .
The formation of a protective film occurs when the preservative solution is kept in the boiler for several days or the solution is circulated through the boiler for several hours.
3.8.5. Methods for preserving turbine units
Preservation with heated air. Blowing the turbine unit with hot air prevents moist air from entering the internal cavities and causing corrosion processes. Moisture ingress on the surfaces of the turbine flow path is especially dangerous if there are deposits of sodium compounds on them. Preservation of a turbine unit with heated air is carried out when it is put into reserve for a period of 7 days or more.
Preservation with nitrogen. By filling the internal cavities of the turbine unit with nitrogen and subsequently maintaining a small excess pressure, the ingress of moist air is prevented. The supply of nitrogen to the turbine begins after the turbine is stopped and the vacuum drying of the intermediate superheater is completed. Nitrogen preservation can also be used for steam spaces of boilers and preheaters.
Preservation of corrosion with volatile inhibitors. Volatile corrosion inhibitors of the IFKHAN type protect steel, copper, and brass by adsorbing on the metal surface. This adsorption layer significantly reduces the rate of electrochemical reactions that cause the corrosion process.
To preserve the turbine unit, air saturated with the inhibitor is sucked through the turbine. Saturation of the air with the inhibitor occurs when it comes into contact with silica gel impregnated with the inhibitor, the so-called linasil. Impregnation of linasil is carried out at the manufacturer. To absorb excess inhibitor, the air at the outlet of the turbine unit passes through pure silica gel. To preserve 1 m 3 of volume, at least 300 g of linasil is required, the protective concentration of the inhibitor in the air is 0.015 g/dm 3.
3.8.6. Conservation of heating networks
When silicate treatment of make-up water is performed, a protective film is formed from the effects of CO 2 and O 2 . In this case, with direct analysis of hot water, the silicate content in the make-up water should be no more than 50 mg/dm 3 in terms of SiO 2.
When treating make-up water with silicate, the maximum calcium concentration should be determined taking into account the total concentration of not only sulfates (to prevent the precipitation of CaSO 4), but also silicic acid (to prevent the precipitation of CaSiO 3) for a given heating temperature of the network water, taking into account the boiler pipes of 40 ° C ( PTE 4.8.39).
With a closed heating system work concentration SiO 2 in the preservative solution can be 1.5 - 2 g/dm 3.
If preservation is not carried out with sodium silicate solution, then heating networks in summer period must always be filled with network water that meets the requirements of PTE 4.8.40.
3.8.7. Brief characteristics of the chemical reagents used
for preservation and precautions when working with them
Aqueous solution of hydrazine hydrate N 2
N 4
N 2
ABOUT
A solution of hydrazine hydrate is a colorless liquid that easily absorbs water, carbon dioxide and oxygen from the air. Hydrazine hydrate is a strong reducing agent. Toxicity (hazard class) of hydrazine – 1.
Aqueous solutions of hydrazine with a concentration of up to 30% are not flammable - they can be transported and stored in carbon steel vessels.
When working with hydrazine hydrate solutions, it is necessary to prevent porous substances and organic compounds from getting into them.
Hoses must be connected to the places where hydrazine solutions are prepared and stored to wash off spilled solutions from equipment with water. To neutralize and render harmless, bleach must be prepared.
Any hydrazine solution that gets on the floor should be covered with bleach and washed off with plenty of water.
Aqueous solutions of hydrazine may cause skin dermatitis and irritate the respiratory tract and eyes. Hydrazine compounds entering the body cause changes in the liver and blood.
When working with hydrazine solutions, you must use personal glasses, rubber gloves, a rubber apron, and a KD brand gas mask.
Drops of hydrazine solution that get on the skin or eyes should be washed off with plenty of water.
Aqueous ammonia solutionN.H. 4
(OH)
An aqueous solution of ammonia (ammonia water) is a colorless liquid with a sharp specific smell. At room temperature and especially when heated, it releases ammonia abundantly. Toxicity (hazard class) of ammonia – 4. Maximum permissible concentration of ammonia in the air – 0.02 mg/dm3. Ammonia solution is alkaline. When working with ammonia it is necessary to carry out the following requirements safety precautions:
– the ammonia solution should be stored in a tank with a sealed lid;
– spilled ammonia solution should be washed off with plenty of water;
– if it is necessary to repair equipment used for preparing and dosing ammonia, it should be thoroughly rinsed with water;
– aqueous solution and ammonia vapor cause eye irritation, respiratory tract, nausea and headache. Getting ammonia into your eyes is especially dangerous;
– when working with ammonia solution, you must use safety glasses;
– ammonia that gets on the skin or eyes must be washed off with plenty of water.
Trilon B
Commercial Trilon B is a white powdery substance.
Trilon solution is stable and does not decompose during prolonged boiling. The solubility of Trilon B at a temperature of 20–40 °C is 108–137 g/dm3. The pH value of these solutions is about 5.5.
Commercial Trilon B is supplied in paper bags with a polyethylene liner. The reagent should be stored in a closed, dry room.
Trilon B does not have a noticeable physiological effect on the human body.
When working with commercial Trilon, you must use a respirator, gloves and safety glasses.
Trisodium phosphateNa 3
P.O. 4
·12N 2
ABOUT
Trisodium phosphate is a white crystalline substance, highly soluble in water.
In crystalline form it has no specific effect on the body.
In a dusty state, if it gets into the respiratory tract or eyes, it irritates the mucous membranes.
Hot phosphate solutions are dangerous if splashed into the eyes.
When carrying out work involving dust, it is necessary to use a respirator and safety glasses. When working with hot phosphate solution, wear safety glasses.
In case of contact with skin or eyes, rinse with plenty of water.
Sodium hydroxideNaOH
Caustic soda is a white, solid, very hygroscopic substance, highly soluble in water (at a temperature of 20 ° C, the solubility is 1070 g/dm3).
Caustic soda solution is a colorless liquid heavier than water. The freezing point of a 6% solution is minus 5 °C, and a 41.8% solution is 0 °C.
Caustic soda in solid crystalline form is transported and stored in steel drums, and liquid alkali in steel containers.
Any caustic soda (crystalline or liquid) that gets on the floor should be washed off with water.
If it is necessary to repair equipment used for preparing and dispensing alkali, it should be washed with water.
Solid caustic soda and its solutions cause severe burns, especially if they come into contact with the eyes.
When working with caustic soda, it is necessary to provide a first aid kit containing cotton wool, a 3% solution of acetic acid and a 2% solution boric acid.
Personal protective equipment when working with caustic soda - a cotton suit, safety glasses, a rubberized apron, rubber boots, rubber gloves.
If alkali gets on your skin, remove it with cotton wool and rinse the affected area. acetic acid. If alkali gets into your eyes
you need to rinse them with a stream of water, and then with a solution of boric acid and go to the first aid station.
Sodium silicate (sodium liquid glass)
Commercial liquid glass is a thick solution of yellow or gray, the SiO 2 content in it is 31 – 33%.
Sodium silicate is supplied in steel barrels or tanks. Liquid glass should be stored in dry, closed areas at a temperature not lower than plus 5 °C.
Sodium silicate is an alkaline product, soluble in water at a temperature of 20 - 40 ° C.
If liquid glass solution gets on your skin, it should be washed off with water.
Calcium hydroxide (lime solution) Ca(OH) 2
Lime mortar is a transparent liquid, colorless and odorless, non-toxic and has a weak alkaline reaction.
A solution of calcium hydroxide is obtained by settling the milk of lime. The solubility of calcium hydroxide is low - no more than 1.4 g/dm 3 at 25 °C.
When working with lime mortar People with sensitive skin are recommended to wear rubber gloves.
If the solution gets on your skin or eyes, wash it off with water.
Contact inhibitor
Inhibitor M-1 is a salt of cyclohexylamine (TU 113-03-13-10-86) and synthetic fatty acids of the C 10-13 fraction (GOST 23279-78). In its commercial form it is a paste or solid substance from dark yellow to brown color. The melting point of the inhibitor is above 30 °C, the mass fraction of cyclohexylamine is 31–34%, the pH of the alcohol-water solution with a mass fraction of the main substance of 1% is 7.5–8.5; The density of a 3 percent aqueous solution at a temperature of 20 ° C is 0.995 - 0.996 g/dm 3.
M-1 inhibitor is supplied in steel drums, metal flasks, steel barrels. Each package must be marked with the following data: name of the manufacturer, name of the inhibitor, batch number, date of manufacture, net weight, gross.
The commercial inhibitor is a flammable substance and must be stored in a warehouse in accordance with the rules for storing flammable substances. An aqueous solution of the inhibitor is not flammable.
Any inhibitor solution that gets on the floor must be washed off with plenty of water.
If it is necessary to repair the equipment used for storing and preparing the inhibitor solution, it should be thoroughly rinsed with water.
The M-1 inhibitor belongs to the third class (moderately hazardous substances). The maximum permissible concentration in the air of the working area for the inhibitor should not exceed 10 mg/dm 3 .
The inhibitor is chemically stable, does not form toxic compounds in the air and wastewater in the presence of other substances or industrial factors.
Persons working with inhibitors must have a cotton suit or robe, gloves, and a hat.
After finishing work with the inhibitor, you must wash your hands. warm water with soap.
Volatile inhibitors
Volatile atmospheric corrosion inhibitor IFKHAN-1(1-diethylamino-2 methylbutanone-3) is a transparent yellowish liquid with a pungent, specific odor.
The liquid inhibitor IFKHAN-1 is classified as a highly hazardous substance in terms of the degree of impact. The maximum permissible concentration of inhibitor vapors in the air of the working area should not exceed 0.1 mg/dm 3 . The IFKHAN-1 inhibitor in high doses causes stimulation of the central nervous system, irritating the mucous membranes of the eyes and upper respiratory tract. Prolonged exposure of unprotected skin to the inhibitor may cause dermatitis.
The IFKHAN-1 inhibitor is chemically stable and does not form toxic compounds in air and wastewater in the presence of other substances.
Liquid inhibitor IFKHAN-1 is a flammable liquid. The ignition temperature of the liquid inhibitor is 47 °C, the auto-ignition temperature is 315 °C. When a fire occurs, the following fire extinguishing agents are used: fire felt, foam fire extinguishers, DU fire extinguishers.
Cleaning of premises should be carried out using a wet method.
When working with the IFKHAN-1 inhibitor, it is necessary to use personal protective equipment - a suit made of cotton fabric (robe), rubber gloves.
Inhibitor IFKHAN-100, also a derivative of amines, is less toxic. A relatively safe exposure level is 10 mg/dm3; ignition temperature 114 °C, self-ignition temperature 241 °C.
Safety measures when working with the IFKHAN-100 inhibitor are the same as when working with the IFKHAN-1 inhibitor.
It is prohibited to carry out work inside the equipment until it is re-opened.
At high concentrations of inhibitor in the air or if it is necessary to work inside the equipment after its re-opening, a gas mask of grade A with a filter box of grade A should be used (GOST 12.4.121-83 and
GOST 12.4.122-83). The equipment should be ventilated first. Work inside the equipment after re-preservation should be carried out by a team of two people.
After finishing working with the inhibitor, you must wash your hands with soap.
If the liquid inhibitor gets on your skin, wash it off with soap and water; if it gets into your eyes, rinse them with plenty of water.
Control questions
Types of corrosion processes.
Describe chemical and electrochemical corrosion.
The influence of external and internal factors on metal corrosion.
Corrosion of the condensate-feed tract of boiler units and heating networks.
Corrosion of steam turbines.
Corrosion of equipment in the make-up and network tracts of the heating network.
Basic methods of water treatment to reduce the intensity of corrosion of heating systems.
The purpose of conservation of thermal power equipment.
List the methods of preservation:
B) hot water boilers;
B) turbine units;
D) heating networks.
10. Give a brief description of the chemical reagents used.
Corrosion of screen pipes is most active in places where coolant impurities are concentrated. This includes areas of screen pipes with high thermal loads, where deep evaporation of boiler water occurs (especially if there are porous deposits with low thermal conductivity on the evaporation surface). Therefore, with regard to preventing damage to screen tubes associated with internal corrosion metal, one must take into account the need for an integrated approach, i.e. impact on both water chemistry and combustion conditions.
Damage to screen pipes is mainly of a mixed nature; they can be divided into two groups:
1) Damage with signs of steel overheating (deformation and thinning of pipe walls at the point of destruction; the presence of graphite grains, etc.).
2) Brittle fractures without characteristic signs of metal overheating.
On the inner surface of many pipes there are significant deposits of a two-layer nature: the upper one is weakly adherent, the lower one is scale-like, tightly adhered to the metal. The thickness of the bottom layer of scale is 0.4-0.75 mm. In the damage zone, the scale on the inner surface is destroyed. Near the places of destruction and at some distance from them, the inner surface of the pipes is affected by corrosion pits and brittle microdamages.
The general appearance of the damage indicates the thermal nature of the destruction. Structural changes on the frontal side of the pipes - deep spheridization and decomposition of pearlite, formation of graphite (transition of carbon into graphite 45-85%) - indicate that not only the operating temperature of the screens, but also the permissible temperature for steel is exceeded 20,500 °C. The presence of FeO also confirms high level metal temperatures during operation (above 845 oK - i.e. 572 oC).
Brittle damage caused by hydrogen typically occurs in areas with high heat flows, under thick layers of sediment, and sloping or horizontal pipes as well as in heat transfer areas near weld backing rings or other devices that impede the free movement of flows. Experience has shown that damage caused by hydrogen occurs in boilers operating at pressures below 1000 psi. inch (6.9 MPa).
Damage caused by hydrogen usually results in thick-edged tears. Other mechanisms that contribute to the formation of thick-edged tears are stress corrosion cracking, corrosion fatigue, stress ruptures, and (in some rare cases) extreme overheating. It may be difficult to visually distinguish damage caused by hydrogen damage from other types of damage, but several features can help.
For example, hydrogen damage almost always involves pitting in the metal (see precautions in Chapters 4 and 6). Other types of failure (with the possible exception of corrosion fatigue, which often begins in individual sinks) are usually not associated with severe corrosion.
Pipe failures as a result of hydrogen damage to metal often manifest themselves in the form of the formation of a rectangular “window” in the pipe wall, which is not typical for other types of damage.
To assess the damageability of screen pipes, it should be taken into account that the metallurgical (initial) content of hydrogen gas in pearlite class steel (including Art. 20) does not exceed 0.5-1 cm3/100g. When the hydrogen content is higher than 4-5 cm3/100g, the mechanical properties of steel deteriorate significantly. In this case, one must focus primarily on the local content of residual hydrogen, since in the case of brittle fractures of screen pipes, a sharp deterioration in the properties of the metal is observed only in a narrow zone along the cross-section of the pipe, with the structure and mechanical properties of the adjacent metal invariably satisfactory at a distance of only 0.2-2 mm.
The obtained values of average hydrogen concentrations at the edge of destruction are 5-10 times higher than its initial content for station 20, which could not but have a significant impact on the damageability of pipes.
The presented results indicate that hydrogen embrittlement turned out to be a decisive factor in the damageability of the screen tubes of KrCHPP boilers.
It was necessary to further study which factor has a decisive influence on this process: a) thermal cycling due to destabilization of the normal boiling regime in zones of increased heat flows in the presence of deposits on the evaporation surface, and, as a result, damage to the protective oxide films covering it; b) the presence in the working environment of corrosive impurities concentrated in deposits near the evaporation surface; c) the combined action of factors “a” and “b”.
Particularly important is the question of the role of the combustion regime. The nature of the curves indicates the accumulation of hydrogen in some cases near the outer surface of the screen pipes. This is possible primarily if there is a dense layer of sulfides on the specified surface, which are largely impermeable to hydrogen diffusing from the inner to the outer surface. The formation of sulfides is due to: high sulfur content of the burned fuel; throwing a torch onto the screen panels. Another reason for hydrogenation of the metal at the outer surface is the occurrence of corrosion processes when the metal comes into contact with flue gases. As the analysis of external deposits of boiler pipes showed, both of the above reasons usually took place.
The role of the combustion regime is also manifested in the corrosion of screen pipes under the influence of clean water, which is most often observed on high-pressure steam generators. Foci of corrosion are usually located in the zone of maximum local thermal loads and only on the heated surface of the pipe. This phenomenon leads to the formation of round or elliptical depressions with a diameter greater than 1 cm.
Overheating of the metal occurs most often in the presence of deposits due to the fact that the amount of heat received will be almost the same for both a clean pipe and a pipe containing scale; the temperature of the pipe will be different.