What is the mass number of an atom. Atomic mass
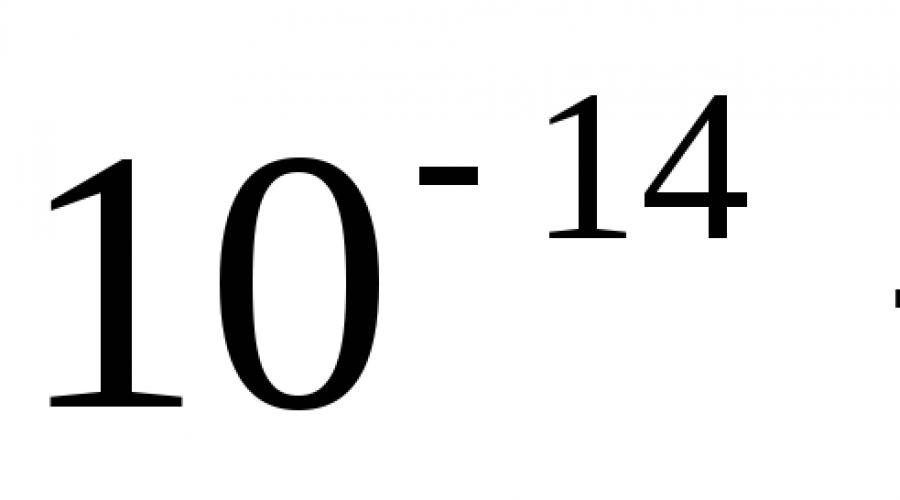
") in the core. Usually denoted by the letter A. The mass number is close to the atomic mass of the isotope, expressed in atomic mass units, but coincides with it only for carbon-12, since the atomic mass unit (a.m.u.) is now defined as 1 ⁄ 12 the mass of an atom of 12 C. In all others cases atomic mass is not an integer, unlike a mass number. Thus, the mass number of the chlorine isotope 35 Cl is 35, and its atomic mass is 34.96885 amu.
The mass number in the designation of a specific nuclide (type of atomic nuclei) is written with an upper left index, for example 232 Th. Nuclides with the same mass number are called isobars (for example, the nuclides 14 C and 14 N are isobars).
Knowing the mass number allows you to estimate the mass of the nucleus and atom. If the mass number is known, then the mass M atom and its nucleus is estimated from the following relation M ≈ A m N, Where m N ≈ 1.67·10 −27 kg - the mass of a nucleon, that is, a proton or neutron. For example, the aluminum-27 atom and its nucleus contain 27 nucleons (13 protons and 14 neutrons). Its mass is approximately 27·1.67·10−27 kg ≈ 4.5·10−26 kg. If it is necessary to obtain the mass of the nucleus with greater accuracy, then it is necessary to take into account that the nucleons in the nucleus are bound by the forces of nuclear attraction, and therefore, in accordance with the relation E=mc 2 the mass of the nucleus decreases. The total mass of electrons in orbits around the nucleus should also be added to the mass of the atom. However, all these corrections do not exceed 1%.
| 238 92 U | → | 234 90 Th | + | 4 2 He |
on the left side the mass number of the initial nucleus is 238, on the right side of the reaction there are two nuclei with mass numbers 234 and 4, which in total gives 238. Taking into account the fact that the mass number of the alpha particle (helium-4 nucleus) is 4, alpha -decay reduces the mass number of the decaying nucleus by 4 units. Any types of beta decay (beta minus decay, positron decay, electron capture, all types of double beta decay) do not change the mass number, since in this process only the transformation of some nucleons of the nucleus from one type to another (protons into neutrons or back). The isomeric transition also does not change the mass number of the nucleus.
Notes
see also
Wikimedia Foundation. 2010.
See what “Mass number” is in other dictionaries:
- (number of nucleons, symbol A), total number NUCLEONS (NEUTRONS and PROTONS) in the ATOMIC NUCLEUS. It is usually written as a superscript before the element's chemical symbol. Thus, the lightest element, hydrogen, has only one proton in its nucleus, and... ... Scientific and technical encyclopedic Dictionary
The total number of nucleons (neutrons and protons) in at. core. Different for isotopes of the same chemical. element. Physical encyclopedic dictionary. M.: Soviet Encyclopedia. Chief Editor A. M. Prokhorov. 1983. MASS NUMBER ... Physical encyclopedia
The number of nucleons in an atomic nucleus. Usually indicated at the top left of the symbol chemical element(eg 10V)… Big Encyclopedic Dictionary
MASS NUMBER- the total number of nucleons (protons and neutrons) in the atomic nucleus, denoted by A and indicated by the index in the upper left of the symbol of the corresponding element, for example. 32S means an isotope of sulfur with a mass number of 32 (A = 32). The part number of the isotope is equal to the whole... ... Big Polytechnic Encyclopedia
mass number- - [Ya.N.Luginsky, M.S.Fezi Zhilinskaya, Yu.S.Kabirov. English-Russian dictionary of electrical engineering and power engineering, Moscow] Topics of electrical engineering, basic concepts EN mass number ... Technical Translator's Guide
The number of nucleons in an atomic nucleus. Usually indicated at the top left of the chemical element symbol (for example, 10B). * * * MASS NUMBER MASS NUMBER, the number of nucleons in an atomic nucleus. Usually indicated at the top left of the chemical element symbol... ... encyclopedic Dictionary
mass number- masės skaičius statusas T sritis Standartizacija ir metrologija apibrėžtis Atomo branduolio nukleonų skaičius. atitikmenys: engl. mass number; nuclear number; nucleon number vok. Massenzahl, f; Nukleonenzahl, f rus. mass number, n; number… … Penkiakalbis aiškinamasis metrologijos terminų žodynas
mass number- masės skaičius statusas T sritis chemija apibrėžtis Atomo branduolio nukleonų skaičius. atitikmenys: engl. mass number; nuclear number; nucleon number rus. mass number... Chemijos terminų aiškinamasis žodynas
mass number- masės skaičius statusas T sritis fizika atitikmenys: engl. mass number; nuclear number; nucleon number vok. Massenzahl, f; Massezahl, f; Nukleonenzahl, f rus. mass number, n pranc. nombre de masse, m; nombre de nucléons, m … Fizikos terminų žodynas
The number of nucleons (protons and neutrons) in an atomic nucleus; denoted by the letter A and is usually indicated at the top left next to the symbol of the element, for example 32S means an isotope of sulfur with A = 32. M.p. and the charge of the nucleus Z, expressed in units of elemental ... Great Soviet Encyclopedia
Books
- Incompetent manager. Incompetence as Mass Madness, Farnham Adrian. A surprising number of people work under managers who are obviously incompetent. Some experts believe that in some sectors of the economy the number of incompetent...
The center of the atom contains the bulk of its mass and all its positive charge. This region of the atom is called the nucleus.
The dimensions of the atom are m, and the dimensions of the nucleus are  m the mass of the nucleus is 99.95% of the mass of the atom. In a neutral atom Z electrons. The nuclear charge is positive and a multiple of the elementary charge
m the mass of the nucleus is 99.95% of the mass of the atom. In a neutral atom Z electrons. The nuclear charge is positive and a multiple of the elementary charge  Cl. The nuclear charge can be represented as
Cl. The nuclear charge can be represented as  , Where Z- charge number, it coincides with the chemical number of the periodic table and is equal to the number of protons included in the nucleus.
, Where Z- charge number, it coincides with the chemical number of the periodic table and is equal to the number of protons included in the nucleus.
The second most important characteristic of a nucleus is its mass. The mass of the nucleus turned out to be greater than the sum of the masses of the protons included in the nucleus.
It was assumed that the nucleus contains neutral particles. In 1932, Chadwig discovered neutrons. Ivanenko and Heisenberg proposed the proton-neutron theory of the nucleus. The nucleus splits into protons and neutrons. They are called nucleons. Total number of nucleons in the nucleus called mass numberA
.
The total number of neutrons is N=A-Z. The rest mass of a proton is  kg, the neutron mass is
kg, the neutron mass is  kg.
kg.
The nucleus of a chemical element is designated by the same symbol as a neutral atom  , Where Z- atomic number (nuclear charge), A- mass number (number of nucleons in the nucleus). Nuclei with the same charge number, but different masses, are called isotopes (isotopes differ in the number of neutrons). Nuclei with the same mass number, but different charge numbers, are called isobars.
, Where Z- atomic number (nuclear charge), A- mass number (number of nucleons in the nucleus). Nuclei with the same charge number, but different masses, are called isotopes (isotopes differ in the number of neutrons). Nuclei with the same mass number, but different charge numbers, are called isobars.
28. Properties of nuclear forces.
Features of nuclear forces:

29. Radioactivity. Alpha and beta decay. Offset rules.
Radioactivity is called the transformation of unstable isotopes of one chemical element into isotopes of another element, accompanied by the emission of some particles. Natural radioactivity is the radioactivity observed in unstable isotopes existing in nature. Artificial radioactivity is the radioactivity of isotopes obtained as a result of nuclear reactions.
Radioactive radiation has a complex composition. In a magnetic field, a narrow beam of radioactive radiation is split into three components:

 -particles –
flow of helium nuclei with charge Z=2
e and mass number A=4
(
-particles –
flow of helium nuclei with charge Z=2
e and mass number A=4
( ). Speed
). Speed  -particles is equal to
-particles is equal to  m/s. Getting into the substance
m/s. Getting into the substance  -particles actively interact with atoms and molecules, ionize and excite it. When the energy
-particles actively interact with atoms and molecules, ionize and excite it. When the energy  -particles are reduced to thermal movement, it captures two electrons and turns into a helium atom ( He). Before this, it goes through a path called a run. Due to the strong interaction with matter, the range is short. A piece of paper or clothing is holding up
-particles are reduced to thermal movement, it captures two electrons and turns into a helium atom ( He). Before this, it goes through a path called a run. Due to the strong interaction with matter, the range is short. A piece of paper or clothing is holding up  -particles. A 0.05 mm thick aluminum sheet also delays
-particles. A 0.05 mm thick aluminum sheet also delays  -particles. Ionizing power
-particles. Ionizing power  -particles are large and equal
-particles are large and equal  steam along the run length.
steam along the run length.
 -particles
is a stream of electrons escaping from nuclei at a speed
-particles
is a stream of electrons escaping from nuclei at a speed  m/s. The nucleus emits an electron when a neutron turns into a proton:
m/s. The nucleus emits an electron when a neutron turns into a proton:
![]()
Where  - electron designation,
- electron designation,  - electron antineutrino.
- electron antineutrino.
Ionizing power  - particles are hundreds of times smaller than those of
- particles are hundreds of times smaller than those of  -particles, and the penetrating power is greater.
-particles, and the penetrating power is greater.  -radiation is delayed by a 2mm thick layer of aluminum.
-radiation is delayed by a 2mm thick layer of aluminum.
What is mass number atomic nucleus? The mass number is numerically equal to the sum of the neutrons and protons of the nucleus. It is designated by the letter A. The concept of “mass number” appeared due to the fact that the mass of the nucleus is determined by the number of nuclear particles. How are the mass of the nucleus and the number of particles related? Let's find out.
Atomic structure
Any atom consists of a nucleus and electrons. Except for the hydrogen atom, since it has only one electron. The nucleus is positively charged. Electrons carry a negative charge. The charge of each electron is taken to be -1. The atom as a whole is electrically neutral, that is, it has no charge. This means that the number of particles carrying a negative charge, that is, electrons, is equal to the positive charge of the nucleus. For example, in an oxygen atom the nuclear charge is +8 and there are 8 electrons; in a calcium atom the nuclear charge is +20 and there are 20 electrons.
Core structure
The nucleus consists of two types of particles - protons and neutrons. Protons are positively charged, neutrons have no charge. Thus, protons give the nucleus a charge. The charge of each proton is taken to be +1. That is, how many protons are contained in the nucleus, this will be the charge of the entire nucleus. For example, there are 6 protons in the carbon nucleus, the charge of the nucleus is +6.
In Mendeleev's periodic table of elements, all elements are arranged in order of increasing nuclear charge. Hydrogen has a +1 nuclear charge and is located first; helium has +2, it is second in the table; lithium has +3, it is third and so on. That is, the charge of the nucleus corresponds to the serial (atomic) number of the element in the table.
In general, any atom is electrically neutral. This means that the number of electrons is equal to the charge of the nucleus, that is, the number of protons. And since the number of protons determines the atomic number of an element, knowing this atomic number, we thus know the number of electrons, the number of protons, and the charge of the nucleus.
Atomic mass
The mass of an atom (M) is determined by the mass of its constituent parts, that is, electrons and nucleus. Electrons are very light compared to the nucleus and contribute almost nothing to the mass of the entire atom. That is, the mass of an atom is determined by the mass of the nucleus. What is mass number? The mass of the nucleus is determined by the number of particles included in its composition - protons and neutrons. Thus, the mass number is the mass of the nucleus, expressed not in mass units (grams), but in the number of particles. Of course, the absolute mass of nuclei (m), expressed in grams, is known. But these are very small numbers, expressed in negative powers. For example, the mass of a carbon atom m (C) = 1.99 ∙ 10 -23 g. It is inconvenient to use such numbers. And if there is no need for absolute values masses, but you just need to compare the masses of elements or particles, then use the relative masses of atoms (A r), expressed in amu. The relative mass of an atom is indicated in the periodic table, for example, nitrogen has 14.007. The relative mass of an atom, rounded to the nearest whole number, is the mass number of the element's nucleus (A). Mass numbers are such that they are convenient to use - they are always integers: 1, 2, 3 and so on. For example, nitrogen has 14, carbon has 12. They are written with an upper left index, for example, 14 N or 12 C.

When do you need to know the mass number?
Knowing the mass number (A) and atomic number of an element in the periodic table (Z), you can determine the number of neutrons. To do this, you need to subtract protons from the mass number.
Knowing the mass number, you can calculate the mass of the nucleus or the entire atom. Since the mass of the nucleus is determined by the mass of the particles that make up its composition, it is equal to the product of the number of these particles and the mass of these particles, that is, the product of the mass of the neutron and the mass number. The mass of a neutron is equal to the mass of a proton; in general, they are designated as the mass of a nucleon (nuclear particle).
For example, let's calculate the mass of an aluminum atom. As can be seen from Mendeleev's periodic table of elements, the relative atomic mass of aluminum is 26.992. Rounding, we get the mass number of the aluminum nucleus to be 27. That is, its nucleus consists of 27 particles. The mass of one particle is a constant value equal to 1.67 ∙ 10 -24 g. Then, the mass of the aluminum core is equal to: 27 ∙ 1.67 ∙ 10 -24 g = 4.5 ∙ 10 -23 g.

What is the mass number of element nuclei you need to know when composing radioactive decay reactions or nuclear reactions. For example, when uranium 235 U captures one neutron 1 n, the nuclei of barium 141 Ba and krypton 92 Kr are formed, as well as three free neutrons 1 n. When composing such reactions, the rule is used: the sum of the mass numbers on the right and left sides of the equation does not change. 235+1 = 92+141+3.
Mass number is the total number of protons and neutrons in the nucleus of an atom. It is designated by the symbol A.
When talking about a specific atomic nucleus, the term nuclide is usually used, and the nuclear particles protons and neutrons are collectively called nucleons.
Atomic number.
The atomic number of an element is the number of protons in the nucleus of its atom. It is denoted by the symbol Z. The atomic number is related to the mass number by the following relationship:
where N is the number of neutrons in the nucleus of an atom.
Each chemical element is characterized by a specific atomic number. In other words, no two elements can have the same atomic number. The atomic number is not only equal to the number of protons in the nucleus of an atom of this element, but is also equal to the number of electrons surrounding the nucleus of an atom. This is explained by the fact that the atom as a whole is an electrically neutral particle. Thus, the number of protons in the nucleus of an atom is equal to the number of electrons surrounding the nucleus. This statement does not apply to ions, which, of course, are charged particles.
The first experimental proof of the atomic numbers of elements was obtained in 1913 by Henry Moseley, who worked at Oxford. He bombarded solid metal targets with cathode rays. (In 1909, Barkla and Kayi had already shown that any solid element, when bombarded with a fast beam of cathode rays, emits X-rays characteristic of that element.) Moseley analyzed the characteristic X-rays using a photographic recording technique. He discovered that the wavelength of characteristic X-ray radiation increases with increasing atomic weight (mass) of the metal and showed that Square root of the frequency of this X-ray radiation is directly proportional to some integer, which he designated by the symbol
![]()
Moseley found that this number was approximately half the value of atomic mass. He came to the conclusion that this number - the atomic number of the element - is fundamental property its atoms. It turned out to be equal to the number of protons in an atom of a given element. Thus, Moseley related the frequency of characteristic X-ray radiation to the serial number of the emitting element (Moseley's law). This law had great importance to establish the periodic law of chemical elements and establish the physical meaning of the atomic number of elements.
Moseley's research allowed him to predict the existence of three missing by that time in periodic table elements with atomic numbers 43, 61 and 75. These elements were discovered later and were named technetium, promethium and rhenium respectively.
Moseley died in the battles of the First World War.
Nuclide symbols.
The mass number of a nuclide is usually indicated as a superscript, and the atomic number as a subscript to the left of the element symbol. For example, the notation means that this carbon nuclide (like all other carbon nuclides) has atomic number 6. This particular nuclide has a mass number of 12. Another carbon nuclide is given the symbol Since all carbon nuclides have atomic number 6, the specified nuclide is often written simply as or carbon-14.
Isotopes.
Isotopes are atomic varieties of one element with different properties. They differ in the number of neutrons in their nucleus. Thus, isotopes of the same element have the same atomic number but different mass numbers. In table Table 1.1 shows the values of the mass number A, atomic number Z and the number of neutrons N in the nucleus of the atoms of each of the three isotopes of carbon.
Table 1.1. Carbon isotopes

Isotopic content of elements.
In most cases, each element is a mixture of different isotopes. The content of each isotope in such a mixture is called isotopic abundance. For example, silicon is found in compounds that occur in nature, with the following natural isotopic abundances: . Please note that the total isotopic abundance of the element must be exactly 100%. The relative isotopic content of each of these isotopes is 0.9228, 0.0467 and 0.0305, respectively. The sum of these numbers is exactly 1.0000.
Atomic mass unit (a.u.m.).
The mass of a nuclide is currently accepted as the standard for defining the atomic mass unit. This nuclide is assigned a mass of 12.0000 amu. Thus, an atomic mass unit is equal to one-twelfth of the mass of that nuclide. The true value of the atomic mass unit is kg. Three fundamental particles, which are components atom have the following masses:
proton mass = 1.007277 amu
neutron mass = 1.008 665 amu
electron mass = 0.000 548 6 a. eat.
Using these values, you can calculate the isotopic mass of each specific nuclide. For example, the isotopic mass of a nuclide is the sum of the masses of 17 protons, 18 neutrons and 17 electrons:
However, accurate experimental data indicate that the isotope mass has a value of 34.968 85 a. e.m. The discrepancy between the calculated and experimentally found values is It is called a mass defect, the reason for the occurrence of a mass defect is explained in section. 1.3.
On the relative atomic mass scale, isotope masses are represented as the result of dividing them by one-twelfth of the mass of the nuclide. Thus, the relative isotopic mass of an isotope is

Note that relative isotopic masses are expressed in dimensionless units.
The relative atomic mass AT of a chemical element is the arithmetic average of the relative isotopic masses, taking into account the isotopic content. It is calculated by summing the products of the relative isotopic mass of each isotope and its relative abundance.
Let's calculate the relative atomic mass of chlorine using the following data:

(called general term"nucleons") in the nucleus. Usually denoted by the letter A. The mass number is close to the atomic mass of the isotope, expressed in atomic mass units, but is the same only for carbon-12, since the atomic mass unit (a.m.u.) is now defined as 1 ⁄ 12 the mass of an atom is 12 C. In all other cases, the atomic mass is not an integer, unlike the mass number. Thus, the mass number of the chlorine isotope 35 Cl is 35, and its atomic mass is 34.96885 amu.
The mass number in the designation of a specific nuclide (type of atomic nuclei) is written with an upper left index, for example 232 Th. Nuclides with the same mass number are called isobars (for example, the nuclides 14 C and 14 N are isobars).
Knowing the mass number allows you to estimate the mass of the nucleus and atom. If the mass number is known, then the mass M atom and its nucleus is estimated from the following relation M ≈ A m N, Where m N ≈ 1.67·10 −27 kg - the mass of a nucleon, that is, a proton or neutron. For example, the aluminum-27 atom and its nucleus contain 27 nucleons (13 protons and 14 neutrons). Its mass is approximately 27·1.67·10−27 kg ≈ 4.5·10−26 kg. If it is necessary to obtain the mass of the nucleus with greater accuracy, then it is necessary to take into account that the nucleons in the nucleus are bound by the forces of nuclear attraction, and therefore, in accordance with the relation E=mc 2 the mass of the nucleus decreases. The total mass of electrons in orbits around the nucleus should also be added to the mass of the atom. However, all these corrections do not exceed 1%.
| 238 92 U | → | 234 90 Th | + | 4 2 He |
on the left side the mass number of the initial nucleus is 238, on the right side of the reaction there are two nuclei with mass numbers 234 and 4, which in total gives 238. Taking into account the fact that the mass number of the alpha particle (helium-4 nucleus) is 4, alpha -decay reduces the mass number of the decaying nucleus by 4 units. Any types of beta decay (beta minus decay, positron decay, electron capture, all types of double beta decay) do not change the mass number, since in this process only the transformation of some nucleons of the nucleus from one type to another (protons into neutrons or back). The isomeric transition also does not change the mass number of the nucleus.
Write a review about the article "Mass number"
Notes
see also
Excerpt characterizing the Mass number
– Tell me, did you not know about the death of the Countess when you stayed in Moscow? - said Princess Marya and immediately blushed, noticing that by making this question after his words that he was free, she ascribed to his words a meaning that they, perhaps, did not have.“No,” answered Pierre, obviously not finding the interpretation that Princess Marya gave to his mention of her freedom awkward. “I learned this in Orel, and you can’t imagine how it struck me.” We were not exemplary spouses,” he said quickly, looking at Natasha and noticing in her face the curiosity about how he would respond to his wife. “But this death struck me terribly.” When two people quarrel, both are always to blame. And one’s own guilt suddenly becomes terribly heavy in front of a person who no longer exists. And then such death... without friends, without consolation. “I’m very, very sorry for her,” he finished and was pleased to notice the joyful approval on Natasha’s face.
“Yes, here you are again, a bachelor and a groom,” said Princess Marya.
Pierre suddenly blushed crimson and tried for a long time not to look at Natasha. When he decided to look at her, her face was cold, stern and even contemptuous, as it seemed to him.
– But did you really see and talk with Napoleon, as we were told? - said Princess Marya.
Pierre laughed.
- Never, never. It always seems to everyone that being a prisoner means being a guest of Napoleon. Not only have I not seen him, but I have also not heard of him. I was in much worse company.
Dinner ended, and Pierre, who at first refused to talk about his captivity, gradually became involved in this story.
- But is it true that you stayed to kill Napoleon? – Natasha asked him, smiling slightly. “I guessed it when we met you at the Sukharev Tower; remember?
Pierre admitted that it was true, and from this question, gradually guided by the questions of Princess Marya and especially Natasha, he became involved in detailed story about your adventures.
At first he spoke with that mocking, meek look that he now had at people and especially at himself; but then, when he came to the story of the horrors and suffering that he had seen, he, without noticing it, became carried away and began to speak with the restrained excitement of a person experiencing strong impressions in his memory.