The acceptor of co2 in c4 of the photosynthetic pathway is. Comparative characteristics of -C3 and -C4 plants. Lipids: classification and their role in the plant
Alternative routes of photosynthesis.
With 3-way turned out to be not the only one in the transformation of Co 2 but in the transformation in the dark phase. Changes in external climate conditions and the composition of the atmosphere in different parts of the Earth caused the emergence of continents with a hot and dry climate (deserts), which could not but affect the plant world. Species specificity is undoubtedly a reflection of the fact that the physiological type of each plant species is unique. Since the 50s, attention has been paid to the processes of photosynthesis, respiration, transpiration, and transport of substances. It turned out that environmental conditions determine the structural and biochemical types of plant photosynthesis.
At the beginning of the 60s of the 20th century, it was found that some plants growing in the tropics or emerging from there, such as corn, sugar cane, sorghum, millet, representatives of the family of goosefoot plants of semi-desert and desert, in total 18 families. Such plants are found in various families and genus, but there is not a single family or genus consisting only of C4 plants. The first products of CO 2 assimilation here have 4 carbon atoms, which is why such plants are called “C4 plants”. In these plants, the stable primary product of assimilated CO 2 is not PHA, but malic acid, and in a number of plants aspartic acid, containing not 3, but 4 carbon atoms, in which the radioactive label 14 CO 2 was discovered (Yu.S. Karpilov, 1960,1969; M.D. Hatch and S.R. Thus, an essentially new path of CO 2 assimilation was discovered, which was called the “C 4 - path” of photosynthesis.
Such plants have a specific anatomical leaf structure. They have two types of photosynthetic cells: sheath cells, which radially cover the vascular bundles in the leaf, and mesophyll cells of columnar and spongy tissue. This can serve as a diagnostic sign for determining C4 plants. There are also differences in the structure of these cells: in the sheath cells of the chloroplasts there are no grana, they are agranal, and in the mesophyll cells all chloroplasts are granal. These two types of cells are not physiologically equivalent and specialize in performing different parts in the conversion of absorbed CO 2
The sheath cells perform the main function; the Calvin cycle occurs in them, in the same way as it occurs in plants with C 3 through photosynthesis, i.e. the entire process of converting carbon dioxide through all stages of the Calvin cycle. Leaf mesophyll cells perform an auxiliary role, pumping CO 2 for the Calvin cycle. Thus, in plants with the C 4 pathway. There are two stages, occurring in different cells of the leaf. For one agranal chloroplast of the sheath cell there are 10 granal mesophyll cells, in which the non-cyclic photophosphorylation pathway occurs. This ensures an increase in photosynthesis even under conditions of very high temperatures and almost closed stomata, which has a positive effect on the water regime of plants, since water consumption is reduced. Both processes are spatially separated, i.e., the assimilation of CO 2 occurs in mesophyll cells, and the formation of sugars and regeneration of the acceptor in the parietal cells. But these two processes occur simultaneously, in the light.
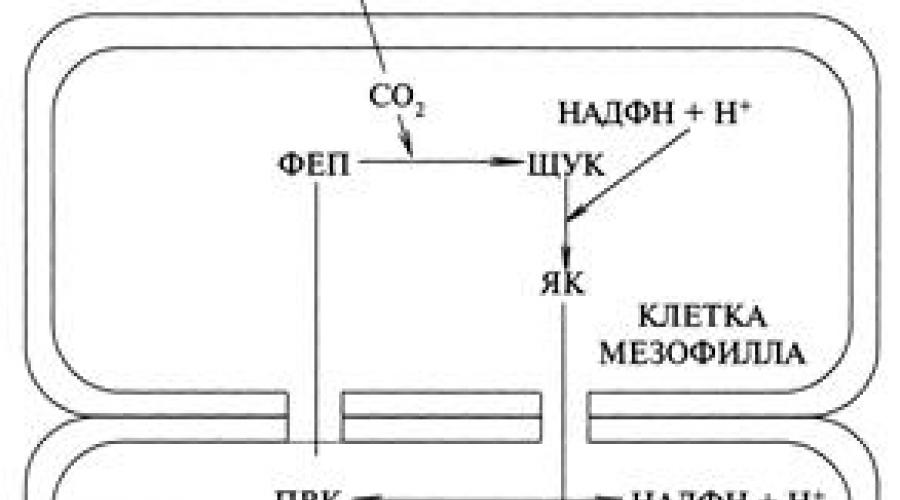
The first reaction of the C 4 cycle - the photosynthesis pathway occurs in the mesophyll of the leaf, in which phosphoenol-pyruvic acid (PEP) is the carbon dioxide acceptor. In these cells, PEP carboxylase is very active, intensively binding CO 2 into an organic compound (Fig.)

Photosynthetic cycle C 4 – photosynthetic pathways
PEP - phosphoenolpyruvic acid;
PCHUK - oxaloacetic acid;
YAC - malic acid;
PVA - pyruvic acid;
RBF - ribulose-1,5-bisphosphate;
3-PGA - phosphoglyceric acid;
PGA - phosphoglyceraldehyde
The reactions proceed as follows. In the mesophyll cells of the leaf, carboxylation of PEP by the enzymes PEP-carboxylase occurs with the formation of PAA oxaloacetic acid: PEP + CO 2 + H 2 O → PEP This unstable substance is stabilized here by NADPH with the formation of malic acid (malate), a 4-carbon compounds: PIKE + NADPH → YAK. In a number of plants, instead of AA, aspartic acid (AA) is formed, which requires ammonia in the cells: PIKE + NH 4 + NADPH → AA. These two acids cannot be formed in the same plant, therefore such plants are called malate or aspartate. The second stage is that malate passes into the lining cells of the vascular bundle, where, as a result of its oxidative decarboxylation, CO 2 and NADPH are formed for the cycle Calvin. The resulting pyruvate (PVC) returns to the mesophyll cells and is phosphorylated into phosphoenolpyruvate, the primary CO 2 acceptor, and the C4 pathway is closed. The released CO 2 goes to the second carboxylation of the RDP of the Calvin cycle with the formation of carbohydrates in the cell linings. Double carboxylation ensures the absorption of CO 2 more intensively than in the C 3 pathway, which is why it is called cooperative. Double carboxylation is much more efficient and provides intense photosynthesis with a reduced CO 2 content. So, if in C 3 plants photosynthesis decreases below 0.03%, then in C 4 plants it occurs fully at 0.008%. The emergence of an alternative pathway for C 4 in plants is considered as a process of evolution of adaptation to a reduced content of CO 2 in the atmosphere and an increase in O 2, due to the plants themselves, than during the period of their emergence. Therefore, these plants have great advantages over the C 3 pathway.
The C4 pathway got its name because in the dark phase, the primary product of CO2 fixation in this case is an organic compound with not three, but four carbon atoms (oxaloacetic acid). Tropical plants of hot countries, for example, bromeliads, have this type of photosynthesis. It has long been noted that these plants absorb CO2 much better than C3 plants. In the anatomical structure of the leaves of C4 plants, along with normal ordinary chloroplasts, around the vascular bundles they have a special type of very dense chloroplasts almost without thylakoids, but filled with starch. These chloroplasts are called parietal chloroplasts.
In ordinary chloroplasts of C4 plants, as expected, the light phase of photosynthesis occurs, and CO2 fixation also occurs, but at the same time oxaloacetic acid is formed. This oxaloacetic acid is converted into malic acid, which enters the parietal chloroplasts, where it is immediately broken down with the release of CO2. And then everything goes the same as with normal C3 plants. In this case, the concentration of CO2 in the parietal chloroplasts as a result becomes significantly higher than in C3 plants, and the very dense arrangement of these chloroplasts ensures that almost no oxygen reaches them; there are no intercellular spaces. Therefore, since there is no oxygen, and there is as much carbon dioxide as you want, photorespiration does not occur.
Thus, in C4 plants, CO2 fixation occurs more efficiently in the form of other compounds, and the formation of sugars occurs in special chloroplasts, resulting in a reduction in the intensity of photorespiration and associated losses.
C4 plants can close their stomata in the heat and not lose such precious moisture. They usually have enough CO2 accumulated in the form of malic acid.
27. Photorespiration: biochemical reactions, their localization. Physiological role of photorespiration.
Photorespiration is a light-activated process of CO2 release and O2 absorption. The primary product of photorespiration is glycolic acid. Photorespiration increases with low CO2 content and high O2 concentration in the air. Under these conditions, chloroplast ribulose disphate carboxylase catalyzes not the carboxylation of ribulose-1,5-diphosphate, but its cleavage into 3-phosphoglyceric and 2-phosphoglycolic acids. The latter is dephosphorylated to form glycolic acid.
Glycolic acid passes from the chloroplast to the peroxisome, where it is oxidized to glyoxylic acid. Glyoxylic acid is aminated to form glycine. Glycine is transported to the mitochondrion, where serine is synthesized from two glycine molecules and CO2 is released.
Serine can enter the peroxisome and transfer the amino group to pyruvic acid to form alanine, and itself is converted to hydroxypyruvic acid. The latter, with the participation of NADPH, is reduced to glyceric acid. It passes into chloroplasts, where it enters the Calvin cycle
In C4 plants-type carbon dioxide released during photorespiration reacts in mesophyll cells with phosphoenolpyruvic acid to form oxaloacetic and malic acids. Malic acid passes into the sheath cells, where it serves as a CO2 donor. C3-path plants characterized by high intensity of photorespiration. Phosphoglycolic acid decomposes through a series of transformations, releasing CO2. Thus, during photorespiration, part of the intermediate products of photosynthesis is lost due to the release of CO2. The oxidation and carboxylation reactions compete with each other, and the implementation of the carboxylase or oxygenase function depends on the content of O2 and CO2
Photorespiration reduces the efficiency of photosynthesis and leads to losses of assimilated carbon, but has some synthetic significance. In the early stages of life, when there was little oxygen in the atmosphere, rubisco occupied a key position in photosynthesis, and its oxygenase function did not cause problems. As the oxygen content increased, the losses due to photorespiration increased, and a number of plants developed mechanisms for the active delivery of rubisco carbon dioxide to the place of work (see C4 and CAM photosynthesis), increasing the share of its carboxylase activity to 100%.
Photosynthesis is the process of transforming the energy of sunlight absorbed by a plant into the chemical energy of organic compounds. From 4 - photosynthesis pathway or Hatch-Slack cycle.
Australian scientists Hatch and Slack described the C4 pathway of photosynthesis, characteristic of tropical and subtropical plants (sugar cane, corn, etc.). The leaves of these plants contain two types of chloroplasts: ordinary ones in the mesophyll cells and large chloroplasts that do not have grana and photosystem II in the sheath cells surrounding the vascular bundles.
In the cytoplasm of mesophyll cells, it adds CO 2 to pyruvic acid, forming oxaloacetic acid. It is transported to chloroplasts, where it is reduced to malic acid with the participation of NADPH. In the presence of ammonium ions, oxaloacetic acid is converted to aspartic acid. Malic and (or) aspartic acids pass into the chloroplasts of the sheath cells and are reduced to pyruvic acid and CO 2 . CO 2 is included in the Calvin cycle, and pyruvic acid is transferred to mesophyll cells, where it is converted into pyruvic acid. This mechanism allows plants to photosynthesize when the stomata are closed due to high temperature. In addition, the products of the Calvin cycle are formed in the chloroplasts of the sheath cells surrounding the vascular bundles. This promotes the rapid outflow of photoassimilates and thereby increases the intensity of photosynthesis.
Lipids: classification and their role in the plant.
Lipids are substances that are quite complex in chemical structure. They also include carbon, oxygen, and hydrogen, but certain groups of lipids may include phosphorus, sulfur, and nitrogen (phosphatides, pigments). All lipids are hydrophobic, i.e. do not dissolve in water. The functions of lipids vary depending on their chemical structure. Lipids are not biopolymers.
Lipids are classified into 5 large groups based on function and structural complexity: Fats - the most easily synthesized group of lipids. From a chemical point of view, these are esters of fatty acids and glycerol. The main functions of fats are energy, construction and storage. Waxes are fat-like substances, solid at room temperature. According to their chemical structure, they are esters between fatty acids and high-molecular monohydric alcohols of the fatty series. The main function of waxes is protective. Phosphatides - which include glycerophosphatides, lecithins and cephalins - are molecules of esters of glycerol, fatty acids and phosphoric acid. These substances are part of storage fats and protect them from rancidity. The main function of phosphatides is storage.
Pigments (chlorophylls and carotenoids) are a special group of lipids with a complex structure, which also includes nitrogen radicals. Pigments include two groups of substances - chlorophylls and carotenoids.
The main function of pigments is participation in the energy (light) phase of photosynthesis. Steroids - these are derivatives of a complex heterocyclic compound . This group of compounds includes high molecular weight alcohols (sterols) and their esters (sterides). The most well-known steroid is ergosterol, from which vitamin D is industrially obtained.
Main function of steroids - construction (participate in the composition of membranes).
UDC 581.1:577.1
EVOLUTIONARY ASPECTS OF C4 PHOTOSYNTHESIS
V.V. Ivanishchev
Issues related to the diversity of manifestations of photosynthetic assimilation of carbon dioxide in plants and the evolutionary aspects of the C4 type of photosynthesis are considered. It is shown that over the past decade and a half, experimental data have been obtained that significantly change our ideas about the ways in which C4 photosynthesis occurs and the implementation of its special properties in a number of typical C3 plants. The prospects for using such knowledge to develop strategies for improving the efficiency of photosynthesis of C¡ plants to increase their productivity are discussed.
Key words: plants, assimilation of inorganic carbon, C3-C4 photosynthesis, C4 photosynthesis, evolutionary aspects, plant productivity.
Introduction
Photosynthesis is the main metabolic pathway through which inorganic carbon is converted into organic compounds on our planet. The study of the mechanisms of the photosynthetic process led to the formation of ideas about the biochemical pathways of assimilation of inorganic carbon of C3 and C4 types. After the discovery of the oxygenase reaction Rubisco f-ribulose-1,5-bisphosphate carboxylase oxygenase, EC 4.1.1.39) it was customary to talk about photorespiration as a parallel process. Individual specialists, incl. The author, who made a significant contribution to the understanding of the biochemistry of photorespiration, began to identify the so-called C2 photosynthesis. Discovered features of transport (not into mitochondria and peroxisomes of the same cell) and metabolism there of phosphoglycolate (formed during the oxidation of D-ribulose-1,5-bisphosphate with oxygen), but its transfer and metabolism in the sheath cells with subsequent “catching” of the released CO2 by chloroplasts These cells made it possible to formulate ideas about C2 photosynthesis. In this case, even the oxygenase reaction of Rubisco is considered as a supplier of CO2, as a result of which the overall efficiency of photosynthesis is at least not greatly reduced.
Active studies of plants whose life is confined to specific environmental conditions over the past three decades have revealed some variations in the manifestation of different pathways of carbon metabolism during photosynthesis. At the same time, high morphological and biochemical plasticity of both C3 and C4 plants was revealed. In addition, a variety of mechanisms were discovered due to which C3-type plants exhibited a number of properties characteristic of C4-type plants.
In the specific ecological conditions of salinity and drought of Central Asia, plants have been discovered that possess a C4-like photosynthetic pathway without the classical Kranz leaf anatomy. Moreover, thanks to another discovery, the sharp line between the two main types of plants in terms of the mechanism of CO2 fixation is erased. It has been shown that in the cells surrounding the central vein of the conducting system of rice leaves (a typical C3 plant), key enzymes characteristic of the C4 type of photosynthesis are present.
All this makes it necessary to generalize the results obtained in order to determine further directions of research in this field of science and possible ways to increase the efficiency of photosynthesis (and productivity) of classical C3 plants, which make up the majority of the most important agricultural crops.
Anatomical and morphological features and plasticity
C4 photosynthesis
Plants with the C4 type of photosynthesis are known to have a whole complex of features. They provide plants with a number of important practical characteristics - greater resistance to certain environmental factors (high temperature, drought), more economical water consumption, greater productivity with less nitrogen consumption, which explains the economic interest of producers in cultivating such crops. The characteristic properties of the leaf structure, first of all, provide C4 plants with a CO2-concentrating mechanism that allows them to prevent the loss of carbon dioxide and thereby reduce photorespiration, due to which the efficiency of photosynthesis in C3 plants can decrease by up to 40%, especially in conditions of high temperature and drought.
In C3 plants, two physiologically and spatially separated types of cells can be observed, namely: one group is more or less evenly distributed and forms the mesophyll, the other part of the cells is concentrated around vascular bundles. This picture makes them look like C4 plants. However, biochemically such cells are the same.
On the other hand, some C3 plants exhibit characteristics of C4 plants in the absence of two anatomically substantially different cell types, consistent with classical ideas of the C4 carbon dioxide fixation pathway. Despite this, C4 photosynthesis is characterized by a number of strictly specific functional features. Understanding evolutionary transformations in this aspect
is based on ideas about changes in cell characteristics and their genetic predetermination.
In most C4 plants, the functioning of PEP carboxylase and the Calvin cycle is spatially separated between these two cell types. The high density of veins of the vascular bundles leads to the fact that the ratio of the volumes of tissues formed by the cells of the mesophyll and the lining of the vascular bundles is approximately 1:1.
The study of the formation of these types of cells shows that during ontogenesis, the inner layers of cells can differentiate from procambium cells, and the outer layers from meristem cells. Phylogenetic analysis shows that C4 plants emerged through several different evolutionary pathways. In this case, the walls of the cells lining the vascular bundles may contain suberin, which limits the diffusion of CO2 beyond the boundaries of such cells. At the same time, a similar layer of suberin is also found in some C3 plants on the outer walls of the inner layers of the lining of the vascular bundles. Thus, no characteristics that are specific only to leaf cells of C4 plants have yet been discovered.
Another important point is the distance between the two types of cells to ensure the maximum rate of exchange of metabolites between them. This explains that the number of layers of the lining cage is limited to one or two. At the same time, an increase in the efficiency of the photosynthesis process is achieved, on the other hand, by a small number of mesophyll cells between the sheath cells, the number of which can be up to four in some species. At the same time, the number of cells between the vascular bundles is generally small. To reduce the distance between the two types of cells, achlorophyll cells are sometimes found in leaves.
At the same time, the study of various dicotyledonous C4 plants showed that, in comparison with typical C3 plants, many of their anatomical and morphological characteristics turned out to be similar. This applies to such indicators as the density of veins per 1 mm2 of leaf surface, leaf thickness, and the percentage of intercellular space. Other indicators had wider or narrower limits of variability, or they were shifted towards higher or lower values.
The third important circumstance characteristic of C4 plants is a large compartment that ensures the maximum possible binding (without loss) of the released CO2 during decarboxylation of the four-carbon compound. This explains the presence of an increased number of chloroplasts present in the sheath cells. The excess of the volume of sheath cells over the volume of mesophyll cells is achieved in several ways, namely: by increasing the number of chloroplasts in sheath cells, increasing the number of sheath cells, or
The number of conducting bundles or changes in the diameter of the veins. The described indicators are interdependent and, in part, determined by environmental conditions, which provide, first of all, the necessary parameters of water exchange and related indicators, such as hydraulic pressure, the rate of flow and movement of water in the plant, etc. All this determines the diversity of the anatomical manifestations of C4 syndrome.
The final feature of C4 plants is that the chloroplasts of the vascular bundle sheath cells and mesophyll cells differ not only in number, but also morphologically and functionally. At the same time, a large number of chloroplasts in sheath cells is characteristic not only of C4 plants, but also of plants with so-called C2 photosynthesis, in which glycine formed during photorespiration is transferred from mesophyll cells to sheath cells, where, CO2 released during its metabolism is further captured by chloroplasts and used in the Calvin cycle. Such special features of the implementation of this type of photosynthesis are considered by researchers as one of the ways through which the evolution of the mechanisms of photosynthetic assimilation of inorganic carbon can be represented in the form of the sequence: C3^C2^C4. Other characteristic features of C4 syndrome include features of the distribution of organelles such as mitochondria and peroxisomes between different cells of plant leaves, as well as features of the ultrastructure and photochemical properties of chloroplasts.
The indicators described above are very flexible. Depending on environmental conditions, they can change significantly, due to which plants with the C3 path of photosynthesis can acquire traits characteristic of the C4 path. At the same time, the variety of possible anatomical and morphological changes in various traits in C4 plants allows them to maintain their physiological and biochemical characteristics at the required level. However, in general, the plasticity of C3 plants in this aspect is assessed higher, despite the fact that experimental data in this area is clearly lacking.
Biochemical features of manifestations of C4 photosynthesis
in higher plants
Classic general ideas about the C4 pathway for inorganic carbon fixation include three main variations in the use of the first stable product - a four-carbon compound (malate and/or aspartate) as a source of CO2 for D-ribulose-1,5-bisphosphate carboxylase oxygenase (Rubisco) - key enzyme in the Calvin cycle. Such forms of plants are designated as NADP-
malik-enzyme, NAD-malik-enzyme and PEP-carboxykinase - named after the key enzyme that ensures the decarboxylation of a four-carbon compound with the release of CO2. Later, 14 varieties of anatomical and biochemical combinations were discovered in C4-type grasses. They differed in such characteristics as the sizes of mesophyll cells and lining cells of vascular bundles, the total volumes of cells of different types, the location of chloroplasts in cells, the presence of the number of grana in chloroplasts, etc. with corresponding variations in cellular distribution and magnitude of enzyme activity.
In contrast to these herbs, only two types of plants are known in dicotyledons: the NADP-malik-enzyme type and the NAD-malik-enzyme type, while PEP carboxykinase is involved in the secondary pathways of carbohydrate metabolism in a number of species. Moreover, the anatomical and biochemical features of such plants are also associated with the environmental features of their growth and development. For example, the study made it possible to identify five varieties of Kranz leaf anatomy in C4 representatives of the Chenopodiaceae family.
A study of the biochemical characteristics of dicotyledonous C4 plants showed that, in general, the described indicators were similar to those characteristic of other C4 species. As a feature, it should be noted once again that PEP carboxykinase does not make a significant contribution to the metabolism of dicarboxylic acids, unlike monocotyledonous grasses. On the other hand, the presence of two decarboxylating enzymes in one species at once (NAD-malik enzyme + PEP carboxykinase or NADP-malik enzyme + PEP carboxykinase) remains largely unclear. This can be partly explained, for example, by the need for PEP synthesis (for the shikimate pathway), saving ATP to support other processes, etc. .
As a result, it was found that among C4-type plants, species with atriplicoid (characteristic of representatives of the genus Atriplex) leaf anatomy and NADP-malik-enzyme biochemistry predominate.
Features of “intermediate” C3-C4 plants
The study of various representatives of the plant kingdom made it possible to talk about a specific photosynthetic group, which began to be designated as C3-C4 plants. The characteristic features of this group (C3-C4 intermediates) are the unique features of gas exchange in leaves, which show a low CO2 compensation point and its curvilinear dependence on the oxygen concentration in the environment. This is due to the special compartmentalization (place of occurrence) of photorespiration in cells without changing photosynthetic carbon metabolism. Majority
representatives of intermediates have C3-type photosynthesis, and their main characteristic is the presence of reduced photorespiration
The anatomical structure of the leaves of C3-C4 intermediates distinguishes them from typical C3 and C4 plants. Unlike C4 plants, in intermediates the mesophyll cells are not concentrated around the lining cells of the vascular bundles and are more loosely located in the leaf structure. At the same time, the intermediate cells contain a large number of organelles, and many of them are located centripetally (toward the center) in the sheath cells. The number of mitochondria and peroxisomes here is four times greater than in mesophyll cells, and mitochondria are twice as large. At the same time, the number of plasmodesmata between the sheath and mesophyll cells is much greater than in C3 plants and approaches the figure characteristic of the C4 type. This ensures rapid exchange of metabolites between different cells of plant leaves.
The key enzyme responsible for the release of CO2, glycine decarboxylase, is mainly localized in the sheath cells. The peculiarities of the relative arrangement of various organelles in cells allow C3 plants to capture up to 50% of the CO2 formed (in glycine conversion reactions), while for C3-C4 intermediates this value can reach 75%.
As a result, these features enable intermediates to exhibit better characteristics in the efficiency of photosynthetic assimilation of CO2, especially at elevated temperatures, in the use of water, etc., which is successfully described using a mathematical model.
The study of the characteristics of biochemistry showed that the intermediates do not have the typical specific distribution of key enzymes (PEP carboxylase and Rubisco) in different cells, as is the case in plants with the C4 type of photosynthesis, show the ineffectiveness of the existing components of the C4 type enzymatic systems, and are characterized by low the activity of enzymes associated with the metabolism of C4-dicarboxylic acids (PEP carboxylase, NADP malate dehydrogenase, NADP malic enzyme, pyruvate orthophosphate dikinase). In this case, significant amounts of labeled carbon from the assimilated 14CO2 end up in the composition of glycine, serine and, especially, fumarate. At the same time, the benefits of some C3-C4 plants are due only to the presence of active glycine exchange between leaf cells, as discussed above. However, active carbon metabolism must be accompanied by an active nitrogen balance, but the picture of this process remains unknown.
C4 photosynthesis without Kranz leaf anatomy
C4 photosynthesis, due to the presence of Kranz anatomy of the leaf, suggests that the cell wall between the sheath and mesophyll cells prevents the loss of CO2 released during decarboxylation of the C4 metabolite. However, the study of plant diversity associated with existence in various ecological niches has made it possible to discover plants in Central Asia in whose leaves many features of C4 photosynthesis are realized within one cell without a fundamental change in leaf anatomy. At the same time, the known data on the mechanisms of CO2 concentration in aquatic plants, as well as the presence of a C4 photosynthetic cycle in some of them within an individual cell, are largely determined by the conditions of existence in the aquatic environment.
In contrast, in land plants there must be a physical boundary (thick cell wall, membrane, envelope) separating the cells where photosynthetic assimilation of carbon dioxide occurs and the cells where the Calvin cycle functions (Fig.).
Simplified scheme of compartmentalization of C^-photosynthesis (two types of cells are shown, between which the assimilation of inorganic carbon and its incorporation are spatially separated
into products of the Calvin cycle)
Terrestrial plants of the CHnporoShasvav family, represented by more than 1300 species, are often resistant to drought and salinity, which is largely ensured by the presence of the C4 type of photosynthesis. Among the representatives of this family, species were discovered in which C4 photosynthesis is realized within one cell without
presence of classical Kranz leaf anatomy. These are Bienertia cycloptera, B. sinuspersici, Suaeda aralocaspica. They grow in conditions of salinity and/or lack of water. At the same time, the sizes of the plants differ significantly, namely: if the height of B. cycloptera and Suaeda aralocaspica is 20 ... 50 cm, then the plants of B. sinuspersici can reach 130 ... 160 cm.
In these species, two types of topographic distribution of organelles within cells were found. Thus, in the cells of X aralocaspica, distal and proximal parts are distinguished. In the distal part, agranal chloroplasts are localized, in which starch is not synthesized, but there is an enzyme - pyruvate orthophosphate dikinase for the synthesis of PEP, and here (in this part of the cell) CO2 is fixed with the formation of a C4 product. In the proximal part of the cell, chloroplasts with enzymes of the Calvin cycle and malik enzyme are concentrated, due to which malate is converted into pyruvate with the release of CO2 for its inclusion in the reaction products of the Calvin cycle with the participation of Rubisco and other enzymes.
In the cells of species of the genus Bienertia, a central and peripheral compartment are distinguished. In the peripheral part there are chloroplasts with a small number of grains, almost no mitochondria, but the key enzymes of the C4 pathway are present - pyruvate orthophosphate dikinase and PEP carboxylase, which ensure the synthesis of PEP and carbon dioxide fixation, respectively. Mitochondria and chloroplasts with a developed granal structure, a large amount of Rubisco and other Calvin cycle enzymes are concentrated in the central part of the cell. In this case, the central compartment is surrounded by a vacuole (vacuoles) to avoid CO2 loss.
At the same time, the described species have different ratios of activities of key enzymes important for the implementation of the C3 and C4 pathways, which distinguishes them from real C4 species.
Another interesting discovery is the discovery of a C4-like type of photosynthesis in the stems and petioles of tobacco, as well as in the midribs of leaves of Arabidopsis (Arabidopsis LaNapa L.) - representatives of dicotyledons. These special features relate to the presence of high activity of all key enzymes of C4 syndrome, incl. the simultaneous presence of high activity of all decarboxylating enzymes (NAD and NADP malic enzymes, as well as PEP carboxykinase).
Later, a similar discovery was made for representatives of monocots. Thus, in cells located around the central vein of the vascular bundle of the leaf of a typical C3 plant - rice, a similar increased activity of the above enzymes was found. The use of immunoblot analysis by the authors gives the obtained
the results have significant significance as an evidence base. It has been shown that Rubisco activity in the cells of this part is two times lower than in other parts of the leaf blade. PEP carboxylase activity is increased by approximately 30%. At the same time, the activity of each of the decarboxylating enzymes (NAD and NADP malic enzymes, as well as PEP carboxykinase) in the midrib region of the leaf was six or more times higher. The activity of another important enzyme, pyruvate orthophosphate dikinase, was 7.5 times higher than that for leaf blade cells.
On the one hand, during the discussion, researchers interpret such data as one of the natural (natural) ways of possible evolution and emergence of C4 photosynthesis, on the other hand, the authors consider such a picture as an opportunity for human manipulation to “improve” the photosynthesis of C3 plants (about what discussions have been going on since the mid-70s of the last century). This applies, first of all, to such a globally significant agricultural crop as rice.
At the same time, the authors of the study devoted a significant portion of their work to studying the peculiarities of the chloroplast pigment system, which provides energy for the synthesis of organic compounds during photosynthesis.
C4 photosynthesis in the light of evolution
The appearance of plants with the C4 type of photosynthesis during evolution, as many authors believe, occurred several dozen times in independent ways. The bulk of such plants today are represented by grasses (approximately 4600 species) and sedges (1600 species), while in dicotyledons, C4-type plants are represented by approximately 1600 species, which suggests a polyphyletic evolutionary origin of C4 syndrome. At the same time, the evolutionary emergence of a new pathway for photosynthetic CO2 assimilation should have included a number of stages affecting the development of Kranz leaf anatomy, the creation of a CO2 pump for concentrating carbon dioxide, the localization and formation of the C4 cycle, changes in gene expression with further optimization of enzyme properties.
A fairly clear and simple theoretical picture of the appearance of C4 photosynthesis, of course, also raises questions regarding changes in the metabolic pathways of other chemical elements and, first of all, nitrogen and sulfur. Therefore, the appearance of cereal plants with C4 syndrome could have occurred quite a long time ago - approximately 30 million years ago, while the appearance of dicotyledons with this type of photosynthesis is estimated at 20 million years.
The emergence of the C4 photosynthetic pathway was previously considered through the appearance of C3-C4 intermediates as transition forms. The study of the variety of features of the arrangement of organelles in the cells of the mesophyll and the lining of the vascular bundles, as well as a number of enzymes, showed that the key mechanism in the appearance of C4 syndrome appears to be the redistribution between different cells of glycine decarboxylase, the activity of which was sharply reduced or lost in mesophyll cells. Indirect evidence of this pathway is that the activity of this enzyme in C4 plants is present only in the sheath cells. At the same time, it remains unclear why in such C3-C4 species, in the presence of a glycine “shuttle” (transfer) from one type of cell to another followed by decarboxylation, the theoretically predicted advantages in the rate of CO2 assimilation and the efficiency of water and nitrogen use do not manifest themselves in conditions of normal carbon dioxide concentrations.
Consideration of the possibilities of anatomical changes in the leaf structure shows that each feature characteristic of the C4 syndrome can change independently in C3 plants (sizes of mesophyll and sheath cells, leaf thickness, distance between the veins of vascular bundles), while for C4 photosynthesis Coordination of such changes is important. From this position, many researchers consider the transition to C4 plants through the emergence of so-called C2 photosynthesis, focusing on the transport of glycine and its decarboxylation in sheath cells.
Thus, the polyphyletic origin of C4 syndrome became possible only due to the emergence of numerous characteristic features, which were coordinated during natural selection under changing environmental conditions.
Prospects for creating concentration mechanisms
CO2 in C3 plants
Theoretical research and a number of experimental data made it possible to talk about the potential genetic transformation of C3 plants, thanks to which it would be possible to improve them (bring them closer to C4 plants in terms of photosynthetic indicators, resistance to environmental factors and productivity). Later, the possibility of a similar mechanism operating only with the participation of oxaloacetate decarboxylase was experimentally substantiated.
Currently, the solution to the problem of increasing the efficiency of photosynthesis (and, as a final result, increasing the productivity) of C3 plants, which represent the bulk of cultivated crops, is considered in the form of several ways.
At the same time, many of the proposed solutions raise reasonable objections.
Of particular interest in this aspect is recent work, which is devoted not only to a detailed analysis of the features of the manifestation of C4 syndrome for understanding the evolution of photosynthetic pathways, but also to the substantiation of the genetic determinism of the evolution of C3 plants into the C4 type. It is noted that until now the genetic mechanisms that ensured the appearance in plants of a specific leaf anatomy characteristic of C4 photosynthesis remain unknown, and this is the main problem in bioengineering attempts to impart new characteristics or transform most of the most important agricultural C3 plants into C4 type.
Features of genetic mechanisms may consist in the fact that many features of C4 photosynthesis are realized, apparently, with the help of many genes, the manifestation of activity of which has small effects, which only collectively give the necessary effect. At the same time, the paths of genetic determinism, as data on various C3-C4- and C4-plants show, can be diverse, since in representatives of individual families and genera the transitional forms of C3-C4-C4-C4 have special features and properties. In this regard, it is first necessary to track such transition features in plant families and genera that are taxonomically close to the corresponding representatives of important agricultural crops.
Problems encountered in transforming C3 plants into the C4 type are described in the work of Gowik and Westhoff. The authors note not only the need to separate reactions involving PEP carboxylase and Rubisco into different cells, but also raise questions about the need for changes in the expression of different genes. This is due, for example, to the fact that the amount of Rubisco in C4 plants is several times lower than in C3 plants. Moreover, the regulatory mechanisms in each type of cell (mesophyll and sheath) differ significantly. Thus, in the C4 plant Flaveria trinervia, a cis-regulatory element is present in the mesophyll cells, which is designated as MESOPHYLL EXPRESSION MODULE1. The same genetic element is also present in the genes of C3-type plants of this genus Flaveria, but it does not have the necessary specificity of manifestation. A slight modification of such an element is sufficient for the specific expression of the specified gene.
In contrast to the above, the specific (for vascular bundle sheath cells) expression of one of the genes encoding the small subunit of Rubisco, FbRbcSl, is regulated mainly at the posttranscriptional level. It seems that the stability of transcripts of this gene in mesophyll and sheath cells is different.
Thus, the transformation of C3 plants into the C4 type is largely associated with changes in the regulation of the activity of various genes. In this case, the key role appears to be played by the GOLDEN2-LIKE transcription factors GLK1 and GLK2, which are characteristic of all land plants. In the Arabidopsis plant, they control the expression of more than 100 genes, many of which are associated with the photosynthetic process, while in maize (C4 plants), sheath cell-specific expression of genes in this group was observed. These results led the authors to conclude that these proteins are important for gene regulation in the formation of mesophyll cells and the lining of vascular bundles. However, further steps may involve optimizing the quantities and properties of many enzymes.
Thus, the evolution of photosynthesis appears to be very complex and diverse, and the problems of bioengineering and control of plant productivity using genetic manipulations seem to have prospects for solution, taking into account the accumulated knowledge about the pathways of regulation of morphological and biochemical characteristics important for the manifestation of a more effective C4 pathway photosynthesis.
Bibliography
1. Heldt G.-W. Biochemistry of plants. M.: BINOM. Knowledge Laboratory, 2011. 471 p.
2. Tolbert N. E. The C2 oxidative photosynthetic carbon cycle // Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997. V. 48. P. 1-25.
3. Lundgren M.R., Osborne C.P., Christin P.-A. Deconstructing Kranz anatomy to understand C4 evolution // J. Exp. Bot. 2014. V. 65 (13). P. 33573369.
4. Proof of C4 photosynthesis without Kranz anatomy in Bienertia cycloptera (Chenopodiaceae) / E.V. Voznesenskaya // The Plant Journal. 2002. V. 31 (5). P. 649-662.
5. Sage R.F. C4 photosynthesis in terrestrial plants does not require Kranz anatomy // TRENDS in Plant Science. 2002. V. 7 (7). P. 283-285.
6. Wickramage A.T. Single cell C4 photosynthesis. 2008. https://www.slideshare.net/anupathisalwickramage/single-cell-c4photosynthesis.
7. The existence of C-4-bundle-sheath-like photosynthesis in the mid-vein of C-3 rice / W.J. Shen // Rice. 2016. V. 9. No. 20. DOI: 10.1186/s12284-016-0094-5.
8. Gowik U., Westholl P. The path from C3 to C4 photosynthesis // Plant Physiol. 2011. V. 155. P. 56-63.
9. Muhaidat R., Sage R.F., Dengler N.G. Diversity of Kranz anatomy and biochemistry in C4 eudicots // Amer. J. Bot. 2007. V.94 (3). P. 362-381.
10. C2 photosynthesis generates about 3-fold elevated leaf CO2 levels in the C3-C4 intermediate species Flaveria pubescens / O. Keerberg //
Journal of Experimental Botany. 2014. V. 65. No. 13. P. 3649-3656. doi:10.1093/jxb/eru239.
11. Glycine decarboxylase is confined to the bundle-sheath cells of leaves of C3-C4 intermediate species / C.M. Hylton // Planta. 1988. V. 175. P. 452-459.
12. Sage R.F., Sage T.L., Kocacinar F. Photorespiration and the evolution of C4 photosynthesis // Annual Review of Plant Biology. 2012. V. 63. P. 19-47.
13. Phenotypic landscape inference reveals multiple evolutionary paths to C4 photosynthesis / B.P. Williams // eLife 2. 2013. e00961. doi.org/10.7554/eLife.00961.
14. Edwards J., Walker D. Photosynthesis of C3 and C4 plants: mechanisms and regulation. M.: Mir, 1986. 590 p.
15. Monson R.K. CO2 assimilation in C3-C4-intermediate plants // Photosynthesis: Physiology and Metabolism / R.C. Leegood, T.D. Sharkey, S. von Caemmerer (eds). Kluwer Academic Publishers. 2000. P. 533-550.
16. Sage R.F. The evolution of C4 photosynthesis // New Phytologist. 2004. V. 161. P. 341-370.
17. Hunt S., Smith A.M., Woolhouse H.W. Evidence for a light-dependent system for reassimilation of photorespiratory which does not include a cycle, in the intermediate species Moricandia arvensis // Planta. 1987. V. 171. P. 227-234.
18. von Caemmerer S. A model of photosynthetic assimilation and carbon-isotope discrimination in leaves of certain intermediates // Planta. 1989. V. 178. P. 463-474.
19. Ivanishchev V.V. On determining the efficiency of carboxylation in plants that have a mechanism for concentrating CO2 // Plant Physiology. 1992. T. 39. No. 3. P. 437444.
20. Hibberd J.M., Quick W.P. Characteristics of C4 photosynthesis in stems and petioles of C3 flowering plants // Nature. 2002. V. 415 (6870). P. 451-454. doi:10.1038/415451a.
21. C4 acid decarboxylases required for C4 photosynthesis are active in the mid-vein of the C4 species Arabidopsis thaliana, and are important in sugar and amino acid metabolism / N.J. Brown // Plant J. 2010. V. 61(1). P. 122-133. doi:10.1111/j.1365-313X.2009.04040.x.
22. Magomedov I.M. On the history of the discovery of C4 photosynthesis. Current state of the problem // Advances in modern natural science. 2015. No. 1 (part 6). pp. 962-965.
23. Nasyrov Yu.S. Genetics of photosynthesis and selection. M.: Knowledge, 1982. 64 p.
24. Abdullaev A., Gorenkova L.G., Ivanishchev V.V. On the distribution of some enzymes of C4-acid metabolism in rye chloroplasts // Plant Physiology. 1989. T. 36. No. 4. P. 665-668.
25. Ivanishchev V.V. Biological significance of oxaloacetate metabolism in chloroplasts of C3 plants // Plant Physiology. 1997. T. 44. No. 3. P. 462-470.
26. Meyer V., Griffiths H. Origins and diversity of eucariotic CO2-concentrating mechanisms: lessons for the future // J. Exp. Bot. 2013. V. 64. No. 3. P. 769-786.
27. Ivanishchev V.V. Problems of photosynthetic assimilation of inorganic carbon by higher plants // Bulletin of GOU DPO TO "IPK AND PPRO TO". Tula educational space. 2017. No. 3 (in print).
28. Evolution and function of a cis-regulatory module for mesophyllspecific gene expression in the C4 dicot Flaveria trinervia / M. Akyildiz // Plant Cell. 2007. V. 19. P. 3391-3402.
29. Patel M., Siegel A.J., Berry J.O. Untranslated regions of FbRbcS1 mRNA mediate bundle sheath cell-specific gene expression in leaves of a C4 plant // J. Biol Chem. 2006. V. 281. P. 25485-25491.
30. Waters M.T., Langdale J.A. The making of a chloroplast // EMBO J. 2009. V. 28. P. 2861-2873.
Ivanishchev Viktor Vasilievich, Doctor of Biology. Sciences, Art. scientific employee, manager department, [email protected], Russia, Tula, Tula State Pedagogical University. L.N. Tolstoy
EVOLUTIONARY ASPECTS OF C4-PHOTOSYNTHESIS V.V. Ivanishchev
The review deals with the variety of manifestations of photosynthetic assimilation of carbon dioxide in plants and the evolutionary aspects of the C4-type photosynthesis. It is shown that over the last decade and a half, experimental data have been obtained that significantly changes our understanding of the ways of the onset of C4 photosynthesis and the realization of its specific properties in a number of typical C3 plants. The prospects of application such knowledge for developing a strategy for improving the efficiency of photosynthesis of C3 plants are discussed in order to increase their productivity.
Key words: plants, assimilation of inorganic carbon, C3-C4-photosynthesis, C4-photosynthesis, evolutionary aspects, plant productivity.
Ivanishchev Viktor Vasiljevich, Doctor of Biology, Senior Researcher, head of chair, [email protected], Russia, Tula, Tula State Lev Tolstoy Pedagogical University
Since in the Calvin cycle the primary products of the incorporation of inorganic carbon into organic carbon are three-carbon compounds, this process is called the C-3 path of photosynthesis.
It is important to note that six revolutions of the Calvin cycle must occur to synthesize one molecule of glucose. Each turnover uses three molecules of ATP (two to activate two molecules of phosphoglyceric acid and one to regenerate ribulose diphosphate) and two molecules of NADP. H 2 to reduce the acid to an aldehyde. Thus, to synthesize one molecule of glucose, it is extremely important to spend 12 molecules of NADP. H 2 and 18 ATP molecules.
It is important to note that the physiological significance of the Calvin cycle is not only the acceptance of carbon dioxide, but also the creation of a mass of carbohydrate compounds, which are used both for the synthesis of reserve substances and for the creation of chloroplast components and the current metabolism of the cell. .
Most plants absorb inorganic carbon through the Calvin cycle. At the same time, a fairly large group of plants (about 500 species) of tropical origin developed in the process of evolution some modification of the process, assimilating inorganic carbon through the formation as a result of its acceptance of four-carbon connections. These are plants that have adapted to photosynthesis under conditions of high air temperature and excess light, as well as low soil moisture (drought). Among cultivated plants, corn, millet, sorghum, and sugar cane have such a metabolic process. A number of weeds also exhibit this very feature of metabolism (pigweed, millet, acorn grass), etc.
A feature of the anatomical structure of such plants is the presence of two types of photosynthetic cells, which are arranged in the form of concentric circles - cells of the parietal parenchyma and mesophyll, radially located around the vascular bundles. This type of anatomical structure is usually called the kranz type (from the German Kranz - wreath).
This type of metabolism was studied in the 60s of the last century, with the research of Soviet scientists Karpilov, Nezgovorova, Tarchevsky, as well as Australian scientists Hatch and Slack playing a major role. It was they who proposed a complete scheme of the cycle; in this regard, it is customary to call this process also the Hatch-Slack-Karpilov cycle.
The process occurs in two stages: CO 2 entering the mesophyll combines with a three-carbon compound (PEP) - phosphoenolpyruvic acid - which is converted into a four-carbon compound. This is the key point in the process, from which it gets its name, since inorganic carbon, when accepted by a three-carbon compound, is converted into a four-carbon compound. Taking into account the dependence on which particular four-carbon compound inorganic carbon is converted into, three groups of plants are distinguished:
NADP-MDH form malic acid with the participation of the enzyme malate dehydrogenase, and then pyruvic acid,
NAD-MDH forms aspartic acid and alanine,
PEP-KK form aspartic acid and phosphoenolpyruvic acid.
The most important plants for agriculture belong to the NADP-MDG type.
After the formation of a four-carbon compound, it moves into the internal cells of the parietal parenchyma and the cleavage or decarboxylation of this molecule occurs. The separated carboxyl group in the form of COO enters the Calvin cycle, and the remaining three-carbon molecule - PEP - returns again to the mesophyll cells.
This way of fixing carbon dioxide allows plants to accumulate carbon reserves in the form of organic acids and carry out the process of photosynthesis during the hottest time of the day while reducing water losses through transpiration due to the closure of stomata. The efficiency of water use by such plants is twice as high as that of plants originating from temperate latitudes.
C4 plants are characterized by the absence of a reverse flow of carbon dioxide during photorespiration and an increased level of synthesis and accumulation of organic substances.